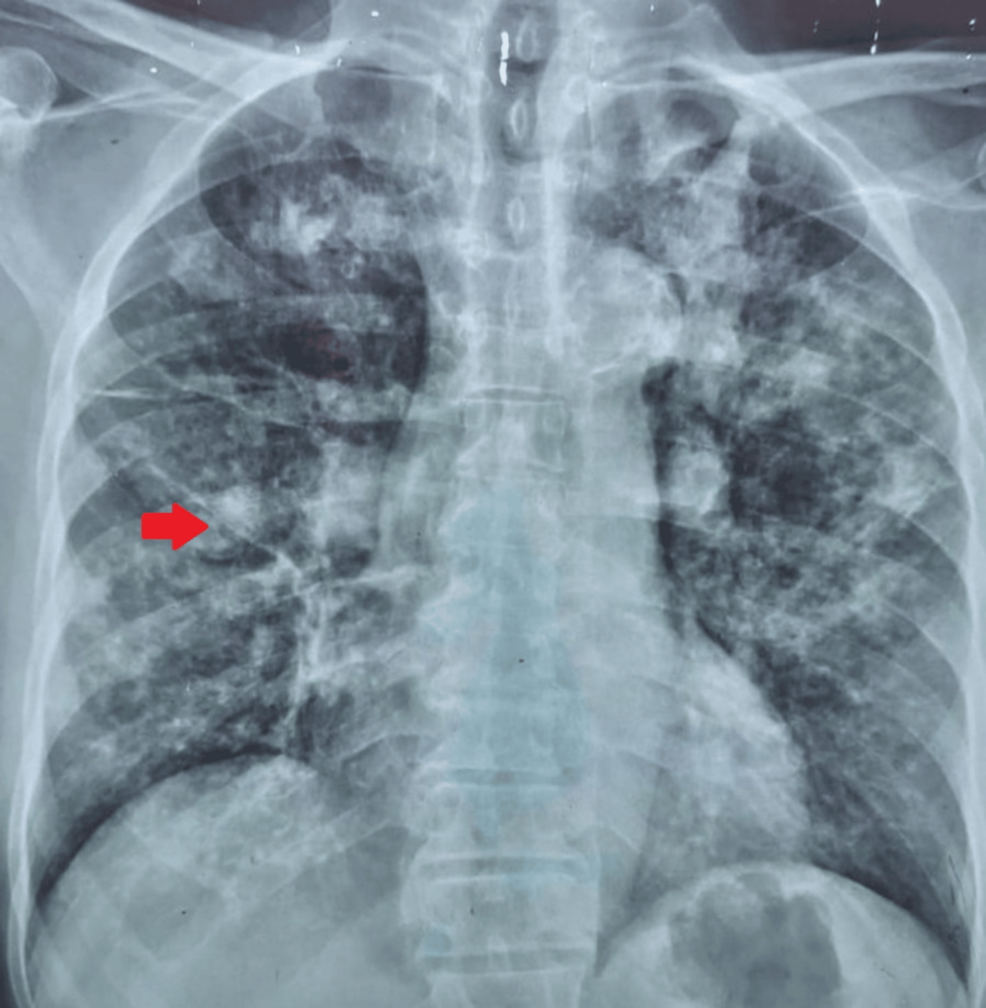
A large review of existing research suggests that tramadol, a strong opioid commonly prescribed for chronic pain, does not provide much meaningful relief. The analysis, published online in BMJ Evidence Based Medicine, found that while tramadol…
Category: 6. Health
-

Remnant cholesterol inflammatory index for Predicting Heart Failure Ri
Background
Coronary artery disease (CAD) and type 2 diabetes mellitus (T2DM) are major global public health concerns, often coexisting and synergistically increasing the risk of adverse cardiovascular events and mortality.1–4 Epidemiological…
Continue Reading
-

Effectiveness Of A Precede–Proceed Model-Based Health Promotion Inte
Introduction
Stunting, a form of chronic malnutrition, remains one of the most pressing public health issues globally and is particularly prevalent in low- and middle-income countries. Characterized by impaired linear growth and cognitive…
Continue Reading
-
Latest Alzheimer Disease Data: Reports from CTAD 2025
Trontinemab Shows Promise for Treatment of Alzheimer Disease in New Data at CTAD
Trontinemab showed a 92% reduction in amyloid plaques, according to new data from the Brainshuttle AD trial. The drug showed an ability to lower amyloid levels and…
Continue Reading
-

Latest Alzheimer Disease Data: Reports from CTAD 2025
Trontinemab Shows Promise for Treatment of Alzheimer Disease in New Data at CTAD
Trontinemab showed a 92% reduction in amyloid plaques, according to new data from the Brainshuttle AD trial. The drug showed an ability to lower amyloid levels and…
Continue Reading
-
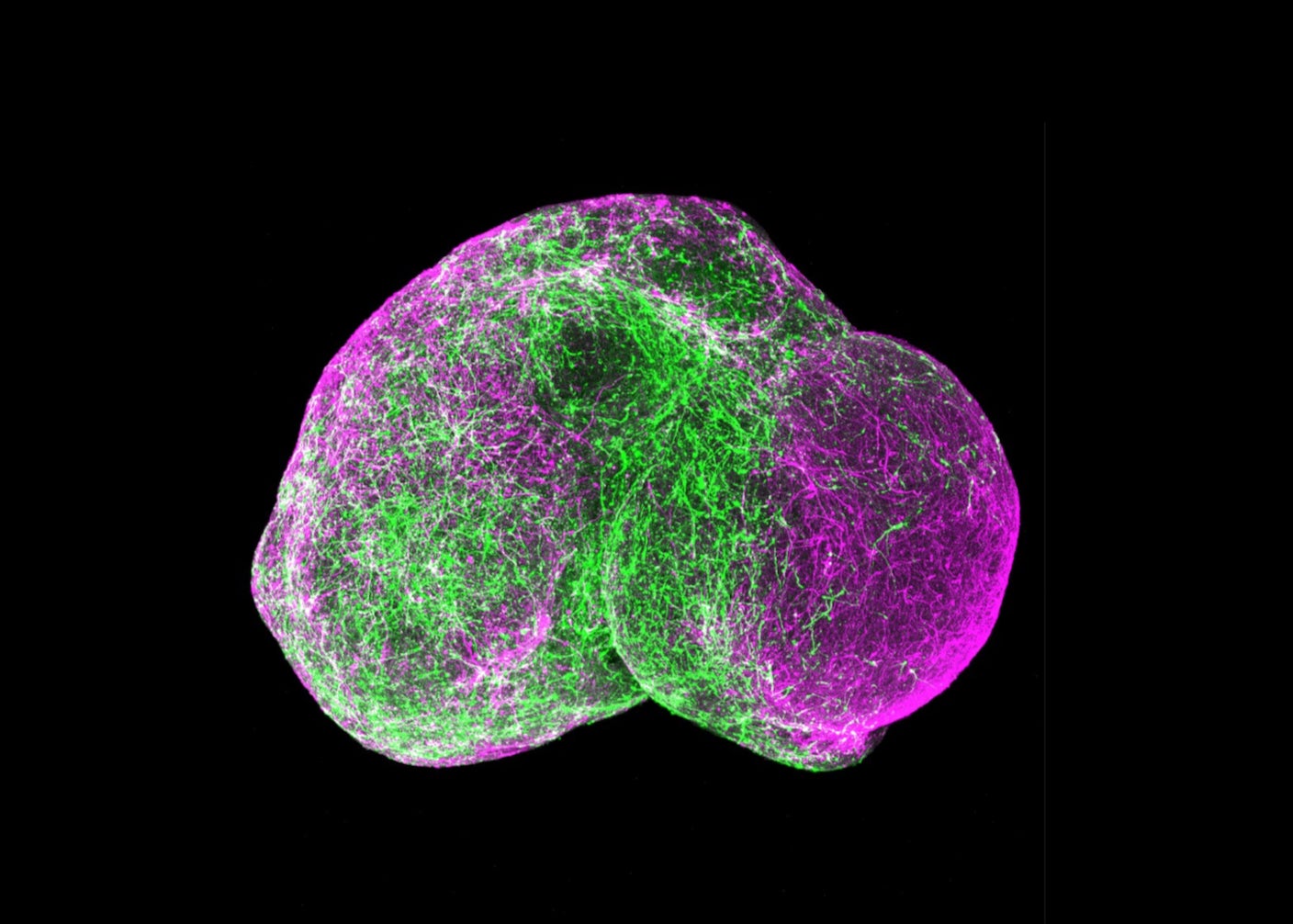
Five-Year-Old Mini Brains Can Now Mimic a Kindergartener’s Neural Wiring. It’s Time to Talk Ethics.
When brain organoids were introduced roughly a decade ago, they were a scientific curiosity. The pea-sized blobs of brain tissue grown from stem cells mimicked parts of the human brain, giving researchers a 3D model to study, instead of the usual…
Continue Reading
-

Psychologists Tap Into a Specific Mindset to Stay Positive, Study Finds : ScienceAlert
Positive psychology forms the backbone of wellbeing programmes around the world. Many people aiming to improve their mental health and live a good life are told to follow a programme of activities that focus on making an intentional effort to…
Continue Reading