A 5-year-old girl named Xiaoni, who underwent two high-risk cardiac surgeries over 110…
Category: 6. Health
-
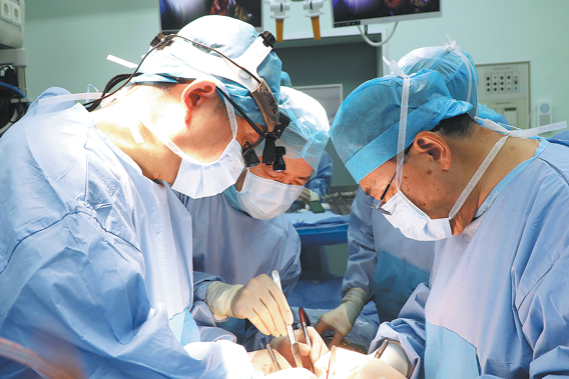
Lifesaving surgery for girl, 5, gives 2nd chance
Doctors perform a cardiac surgery on Xiaoni at TEDA International Cardiovascular Hospital in Tianjin. China Daily
-
Bow Wow Lounge rabies: Chicago dog daycare says dog with virus visited facility; 1st case in decades, IDPH says
CHICAGO (WLS) — For the first time in decades, a dog in Chicago tested positive for rabies.
ABC7 Chicago is now streaming 24/7. Click here to watch
The Bow Wow Lounge, a pet boarding and daycare facility on North Ravenswood said the rabid dog,…
Continue Reading
-

Orthopaedic experts share 5 foods to prevent vitamin D deficiency and maintain bone health during sun-deprived winters
Reduced sunlight exposure during winters quietly pushes a large section of the population toward vitamin D deficiency. While the issue is often underestimated, its consequences are far-reaching – ranging from bone pain and muscle weakness to
Continue Reading
-
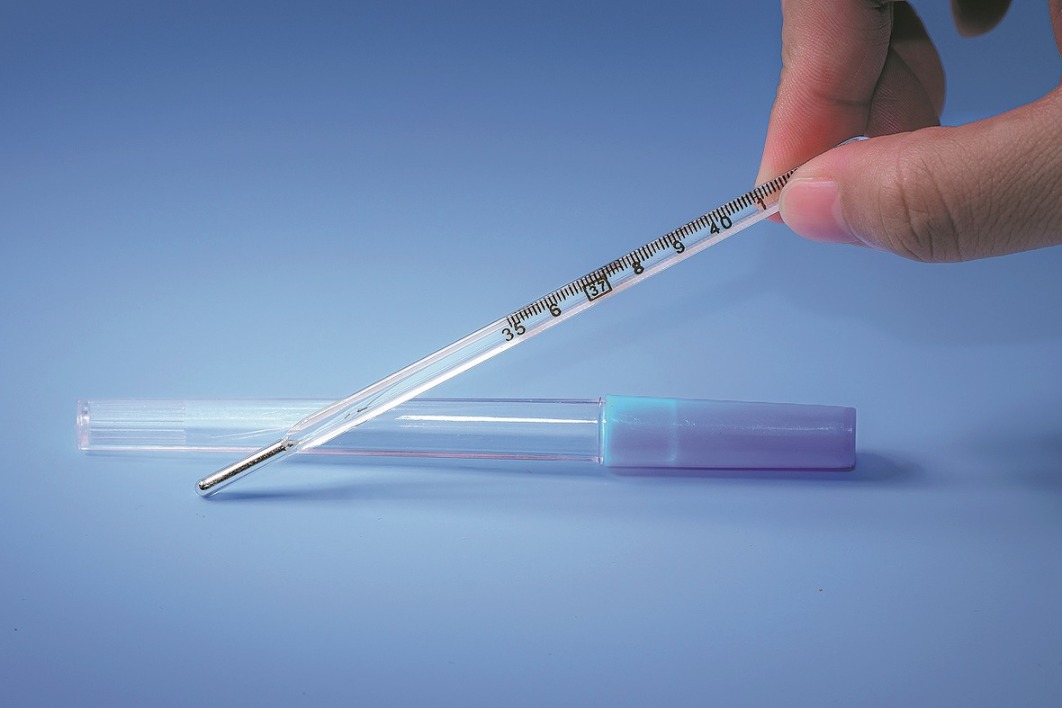
Ban on mercury thermometers to take effect soon
China will ban mercury thermometers from Jan 1. HUANG TAIMING/FOR CHINA DAILY
China will ban the production of mercury-containing thermometers and blood pressure monitors from Jan 1, aiming to curb mercury…
Continue Reading
-

Scientists find a way to develop crops that can withstand eco-stress
An international team of researchers has uncovered a genetic secret within barley seeds that could help protect the global food supply from the unpredictable effects of climate change. The study, published on Nov 6 in…
Continue Reading
-

Ban on mercury thermometers to take effect soon
China will ban mercury thermometers from Jan 1. HUANG TAIMING/FOR CHINA DAILY
China will ban the production of mercury-containing thermometers and blood pressure monitors from Jan 1, aiming to curb…
Continue Reading
-
Epilepsy drug topiramate shows mixed results for treating combined alcohol and tobacco use
An 18-week experimental study examining the effects of topiramate on tobacco smoking and alcohol use found no differences between groups treated with topiramate and those receiving placebo treatment in the last 4 weeks of treatment. However, the…
Continue Reading
-
JAK-1 inhibitors in the management of atopic dermatitis in patients over 65 years old: a descriptive cohort study
Chan, L. N. et al. The epidemiology of atopic dermatitis in older adults: A population-based study in the united Kingdom. PloS One. 16, e0258219 (2021).
Silverberg, J. I. Atopic dermatitis in…
Continue Reading
-
Strong policies, digitalization boost immunization program
Lance Rodewald
China”s national immunization system has grown more robust and intelligent in recent years, positioning the country to further expand vaccination coverage and strengthen international…
Continue Reading