This year’s flu season began early, with five children dying from complications of the disease and hospitalizations on the rise. Family doctors are warning that influenza is not a mild winter illness, outlining its symptoms, identifying who is…
Category: 6. Health
-
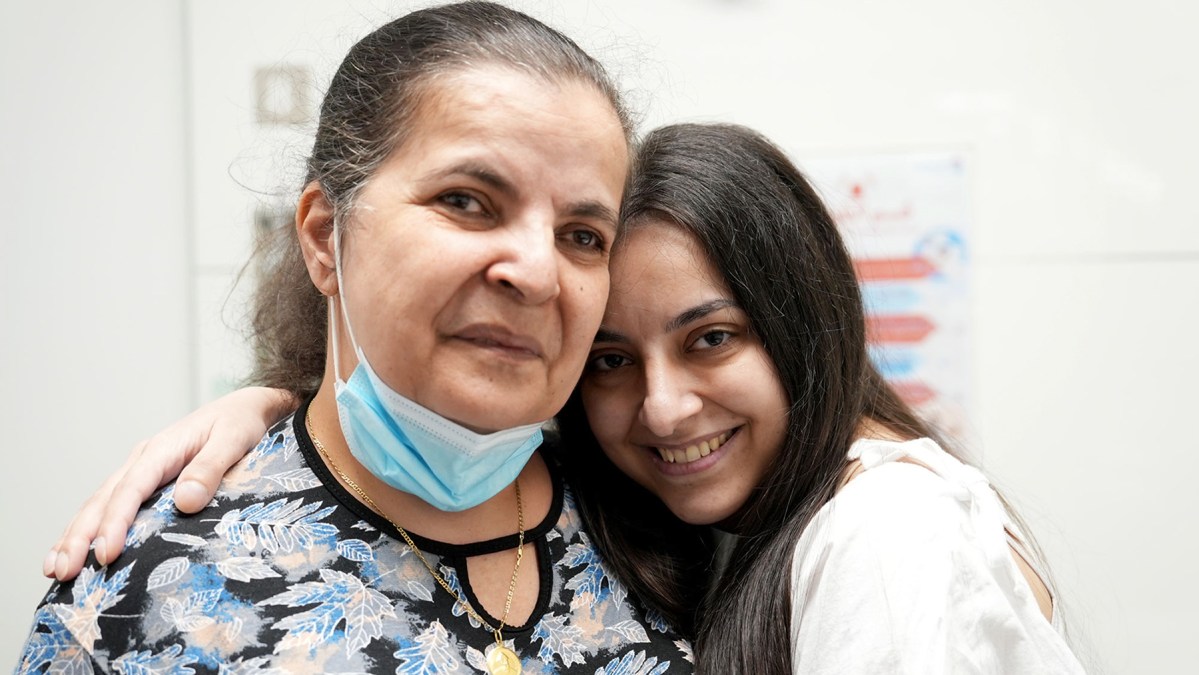
Transforming the Lives of Cystic Fibrosis Patients in Lebanon
By ANERA
Editor’s note: This article was originally published by Anera here.
Cystic fibrosis is a life-threatening genetic disease affecting the lungs and digestive system. In Lebanon, access to treatment is limited, leaving families to face…
Continue Reading
-
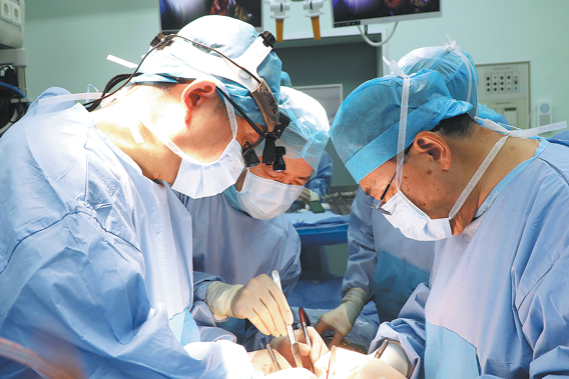
Lifesaving surgery for girl, 5, gives 2nd chance
Doctors perform a cardiac surgery on Xiaoni at TEDA International Cardiovascular Hospital in Tianjin. China Daily
A 5-year-old girl named Xiaoni, who underwent two high-risk cardiac surgeries over 110…
Continue Reading
-
Bow Wow Lounge rabies: Chicago dog daycare says dog with virus visited facility; 1st case in decades, IDPH says
CHICAGO (WLS) — For the first time in decades, a dog in Chicago tested positive for rabies.
ABC7 Chicago is now streaming 24/7. Click here to watch
The Bow Wow Lounge, a pet boarding and daycare facility on North Ravenswood said the rabid dog,…
Continue Reading
-

Orthopaedic experts share 5 foods to prevent vitamin D deficiency and maintain bone health during sun-deprived winters
Reduced sunlight exposure during winters quietly pushes a large section of the population toward vitamin D deficiency. While the issue is often underestimated, its consequences are far-reaching – ranging from bone pain and muscle weakness to
Continue Reading
-
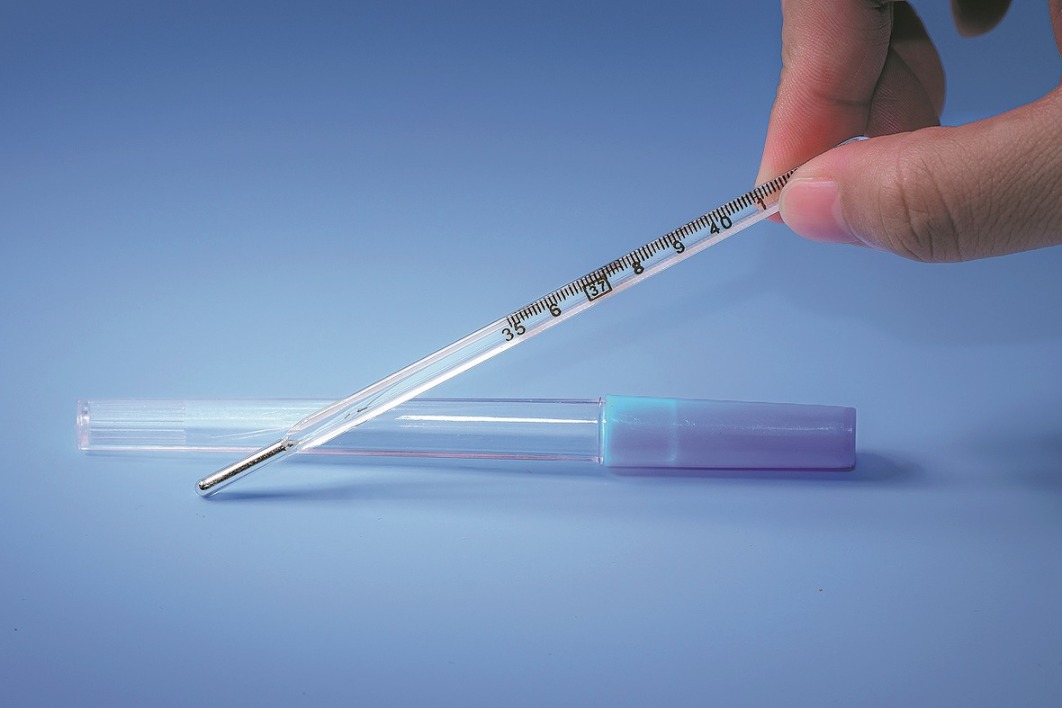
Ban on mercury thermometers to take effect soon
China will ban mercury thermometers from Jan 1. HUANG TAIMING/FOR CHINA DAILY
China will ban the production of mercury-containing thermometers and blood pressure monitors from Jan 1, aiming to curb mercury…
Continue Reading
-

Scientists find a way to develop crops that can withstand eco-stress
An international team of researchers has uncovered a genetic secret within barley seeds that could help protect the global food supply from the unpredictable effects of climate change. The study, published on Nov 6 in…
Continue Reading
-

Ban on mercury thermometers to take effect soon
China will ban mercury thermometers from Jan 1. HUANG TAIMING/FOR CHINA DAILY
China will ban the production of mercury-containing thermometers and blood pressure monitors from Jan 1, aiming to curb…
Continue Reading
-
Epilepsy drug topiramate shows mixed results for treating combined alcohol and tobacco use
An 18-week experimental study examining the effects of topiramate on tobacco smoking and alcohol use found no differences between groups treated with topiramate and those receiving placebo treatment in the last 4 weeks of treatment. However, the…
Continue Reading
