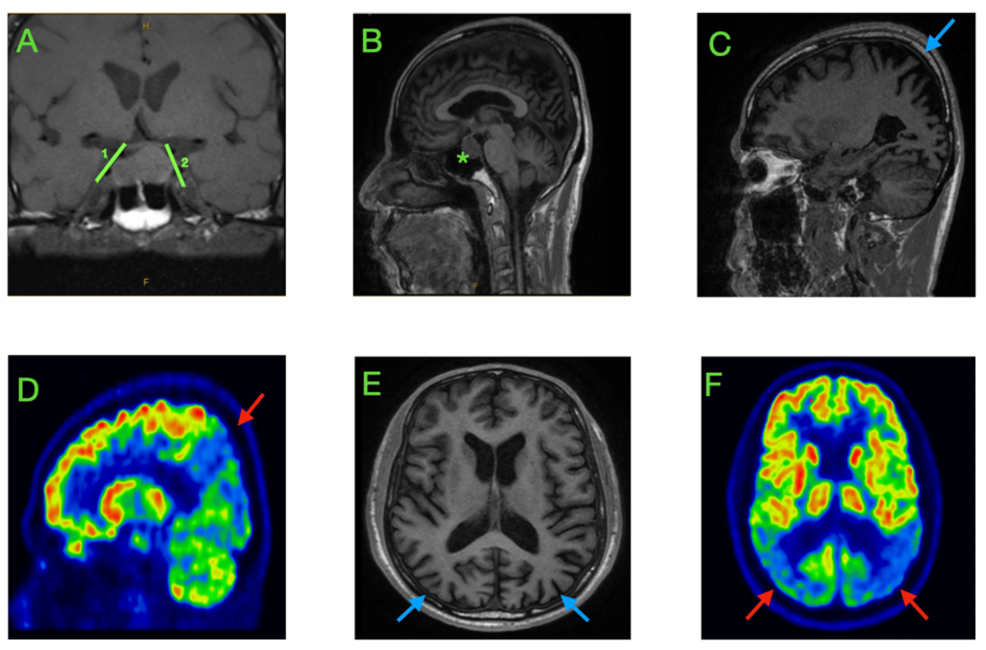
Category: 6. Health
-
Israel may leave WHO, new health committee chair says
Israel may leave WHO, new health committee chair says | The Jerusalem Post -

Young cancer patients receiving Christmas hampers in Jersey
A cancer survivor is helping other young patients going through treatment over Christmas by providing presents for them.
Hampers have been put together for nine people in Jersey after more than £1,100 was raised in online donations as part of the…
Continue Reading
-
Vietnam’s capital records decline in dengue fever cases-Xinhua
HANOI, Dec. 23 (Xinhua) — The number of dengue fever cases in Vietnam’s capital Hanoi has declined in recent weeks, reflecting the effectiveness of preventive measures in curbing the outbreak, Vietnam News Agency reported Tuesday.
From Dec….
Continue Reading
-

Are peer reviewers influenced by their work being cited?
This is an observational study, meaning we cannot rule out unmeasured confounding and should be cautious in interpreting the results.
To our knowledge, this is the first analysis to use a matched design and analysis when examining reviewer…
Continue Reading
-

Antimicrobial Resistance Patterns and Epidemiological Distribution of
Introduction
In the Pediatric Intensive Care Unit (PICU), infectious diseases caused by pathogens have become one of the key causes of rapid deterioration of the condition of critically ill children and even death.1 Such infections not only…
Continue Reading
-
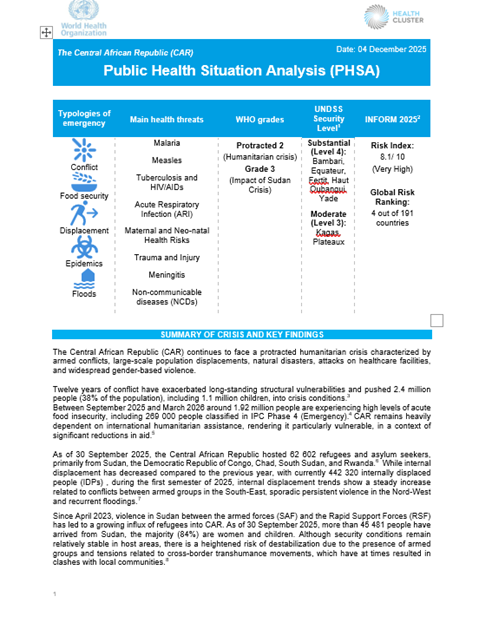
Public Health Situation Analysis – the Central African Republic
Overview
The Central African Republic (CAR) continues to face a protracted humanitarian crisis characterized by armed conflicts, large-scale population displacements, natural disasters, attacks on healthcare facilities, and widespread…
Continue Reading
-
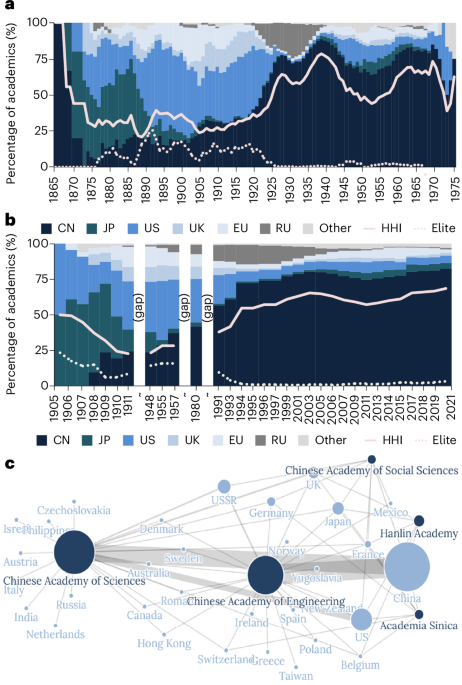
Indigenization and inclusion in Chinese academia
Fewer returnees among regular members despite globalization
Scholars’ educational attainment exposes dynamics and hierarchies in academia, as well as reflecting social-economic roots and geopolitical narratives29,30. By breaking down the data,…
Continue Reading
-
COVID-19 Delays Linked to Advanced Colorectal Cancer Diagnosis
A real-world survey from Japan suggests that pandemic-related behavioral changes contributed to delayed colorectal cancer diagnosis, with patients who postponed medical visits during COVID-19 more likely to present with more advanced disease.
Continue Reading
-

What does a huge festive meal do to your brain?
More like this:
• Why we all enjoy a good feast
• The surprising foods that make you sleep better
• Should you eat in front of the TV?
This study expands on existing research, Kullman says, showing the communication between our guts and…
Continue Reading