Introduction
Vietnam is experiencing one of the fastest rates of population aging globally, with individuals aged 60 and older comprising 11.9% of the population in 2023, projected to reach 17.9% by 2030.1 This demographic shift has amplified…

Vietnam is experiencing one of the fastest rates of population aging globally, with individuals aged 60 and older comprising 11.9% of the population in 2023, projected to reach 17.9% by 2030.1 This demographic shift has amplified…

Meruyet Kuspanova,1 Andrey Gaiday,1 Saule Bermagambetova,1 Andrii Dinets,2,3 Zhanna Amirbekova,4 Gulmira Zhylkaidar,4 Gulnaz Sarkuchikova,5 Svetlana Sakhanova,1 Aruzhan Amantay,1 Meruyert Aidarkhanova,6 Akylbek Tussupkaliyev1
1Department of…
Women with severe coronary heart disease causing narrowing or blockages in the arteries may derive greater long-term benefits from coronary artery bypass grafting compared with percutaneous coronary intervention, also known as…

During the COVID-19 pandemic, disinfectants became our shield. Hand sanitizers, disinfectant wipes and antimicrobial sprays became part of daily life. They made us feel safe. Today, they are still everywhere: in homes, hospitals and public…
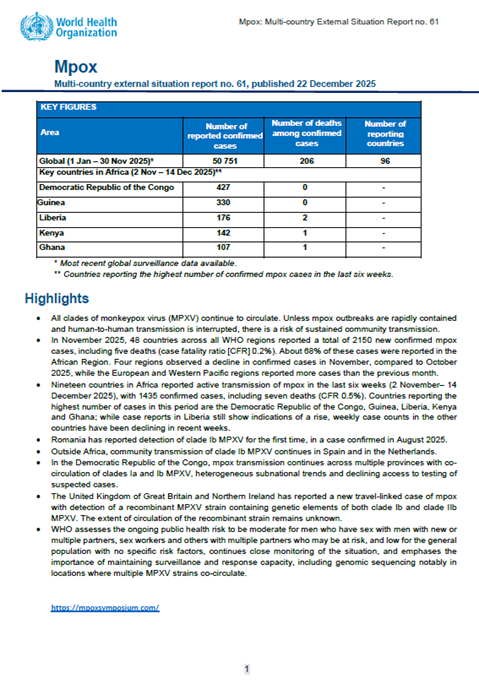
This is the 61th situation report for the multi-country outbreak of mpox, which provides details on the global epidemiological situation for mpox, including an update on the epidemiological situation with data on the global…

Stomach cancer is often overshadowed by more widely discussed cancers, yet it remains a serious disease that affects thousands of people each year. While overall rates of stomach cancer have declined over time, awareness remains critical. This…

Aging is a progressive process characterized by the accumulation of cellular and molecular damage, resulting in functional decline across various physiological systems.1 Substantial efforts have been directed toward developing…

Physicians at the University of Iowa (UI) Health Care System were recently awarded a grant to support a quality improvement initiative focused on identifying patients with prostate cancer who may be eligible for germline genetic testing.1

SARS-CoV-2 is a major public health burden causing morbidity and mortality in the global community and leading to respiratory sequelae, and cardiovascular, neurological, and digestive complications.1,2 Myocardial and cerebral…

The liver plays a crucial role in protein synthesis, glucose and lipid metabolism, and detoxification.1 Metabolic-associated fatty liver disease (MAFLD), previously known as non-alcoholic fatty liver disease (NAFLD), was recently…