Canada lost its measles elimination status on November 10, 2025, after more than 12 months of sustained transmission of the highly contagious and deadly viral disease. The decision by the Pan American Health Organization (PAHO) – which is the…
Category: 6. Health
-
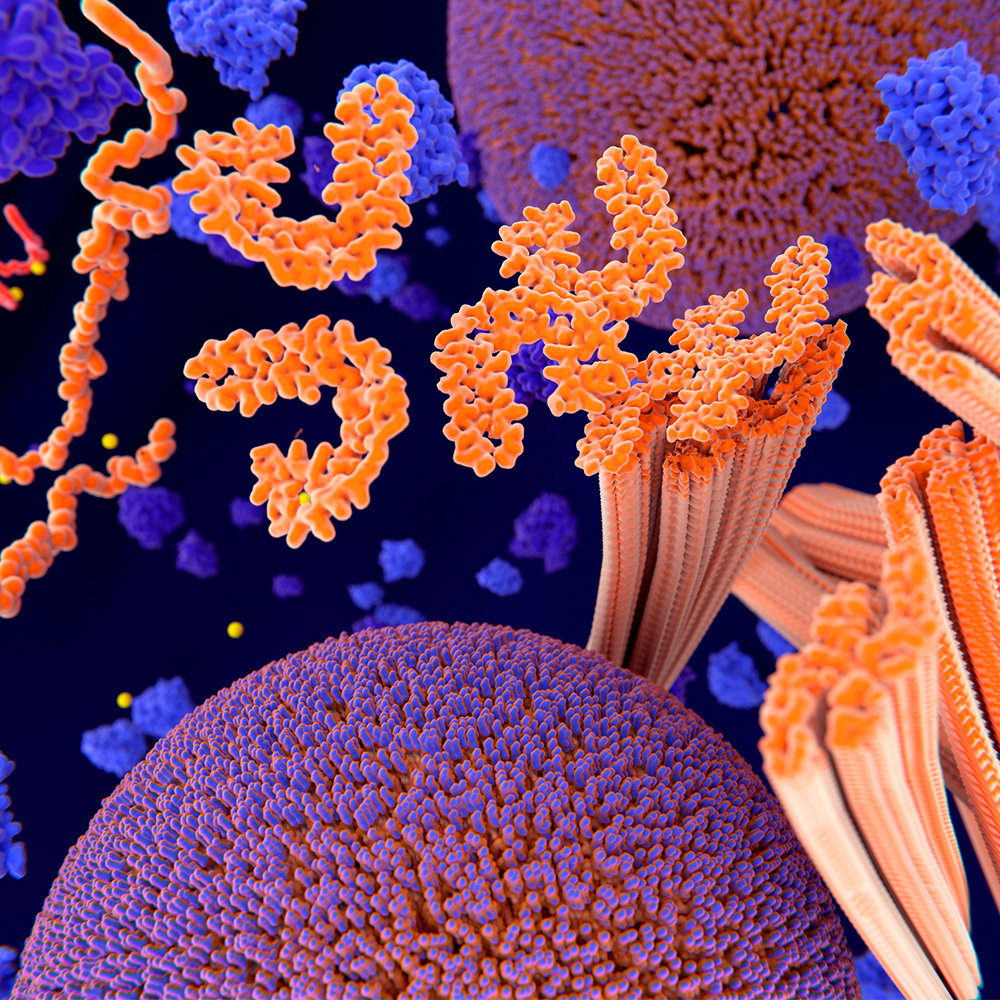
Modified tau thwarts aggregation in neurodegenerative disease: Newsroom
This 3D illustration shows tau proteins (orange on left), which play an essential role in cells but can aggregate (orange C-shaped tubes at center and right), forming harmful deposits in the brain and causing…
Continue Reading
-
Vitamin D Deficiency Affects Bone Health and Immune Function in Many Americans – geneonline.com
- Vitamin D Deficiency Affects Bone Health and Immune Function in Many Americans geneonline.com
- Lacking This 1 Vitamin Might Be The Reason You’re Sick All The Time HuffPost
- Not only a ‘sunshine vitamin’: Why low Vitamin D can worsen Diabetes and…
Continue Reading
-
Bypass Surgery May Offer Greater Long-Term Benefits Compared with Stents for Women | Newsroom
Women with severe coronary heart disease causing narrowing or blockages in the arteries may derive greater long-term benefits from coronary artery bypass grafting compared with percutaneous coronary intervention, also known as…
Continue Reading
-

Long-Term ART Lowers Pneumonia and Shingles Risk in HIV – European Medical Journal Community-Acquired Pneumonia and Herpes Zoster in HIV
Infection Risk Patterns in the ART Era
People with HIV on antiretroviral therapy (ART) still face elevated risks of community-acquired pneumonia and herpes zoster, particularly when immune recovery remains incomplete, according to a large…
Continue Reading
-
Robotic Prostatectomy Lowers Early Complications
A LARGE nationwide French cohort study has found that robot-assisted radical prostatectomy (RARP) is associated with significantly fewer short-term postoperative complications compared with both open and laparoscopic approaches. The findings…
Continue Reading
-
We dodged a vaccine disaster (for now). And a quick virus weather report before the break.
Happy holidays! I have never needed a break more than I do after this year, so YLE will be off for the next two weeks.
Since it’s a holiday week, this Dose was supposed to be a light one, with a quick infectious disease “weather report” to…
Continue Reading
-

The ABCs of Vitamin D Supplements: Exploring Their Health Benefits and Proper Use
According to the National Institutes of Health Office of Dietary Supplements (NIHODS), vitamin D, also known as calciferol, is a fat-soluble nutrient naturally found in a limited number of foods, such as egg yolks, beef liver, and fatty fish….
Continue Reading
-

Epilepsy Foundation of America Announces Lampsy Health as Winner of 2025 Startup Accelerator Course
BOWIE, Md., Dec. 22, 2025 /PRNewswire/ — The Epilepsy Foundation of America (EFA), in partnership with the Danny Did Foundation, announced Lampsy Health as the winner of the 2025…
Continue Reading
-

Neurofeedback and deep brain reorienting
Dr. Ruth Lanius, Scientist at London Health Sciences Centre Research Institute (LHSCRI) and Psychiatrist at London Health Sciences Centre (LHSC) discusses the need for novel adjunct treatments for posttraumatic stress disorder (PTSD),…
Continue Reading