Having had a stroke caused by blocked blood vessels (ischemic stroke) more than doubled an expectant mother’s odds of having another stroke during pregnancy and within six weeks of childbirth, according to a preliminary study to…
Category: 6. Health
-
NYUAD researchers develop nanotechnology to improve cancer detection, treatment
– Advertisement –
– Advertisement –
– Advertisement –
ABU DHABI, Jan 29 (WAM/APP): Researchers at NYU Abu Dhabi (NYUAD) have developed a new light-based nanotechnology that could improve how certain cancers are detected and treated, offering a…
Continue Reading
-
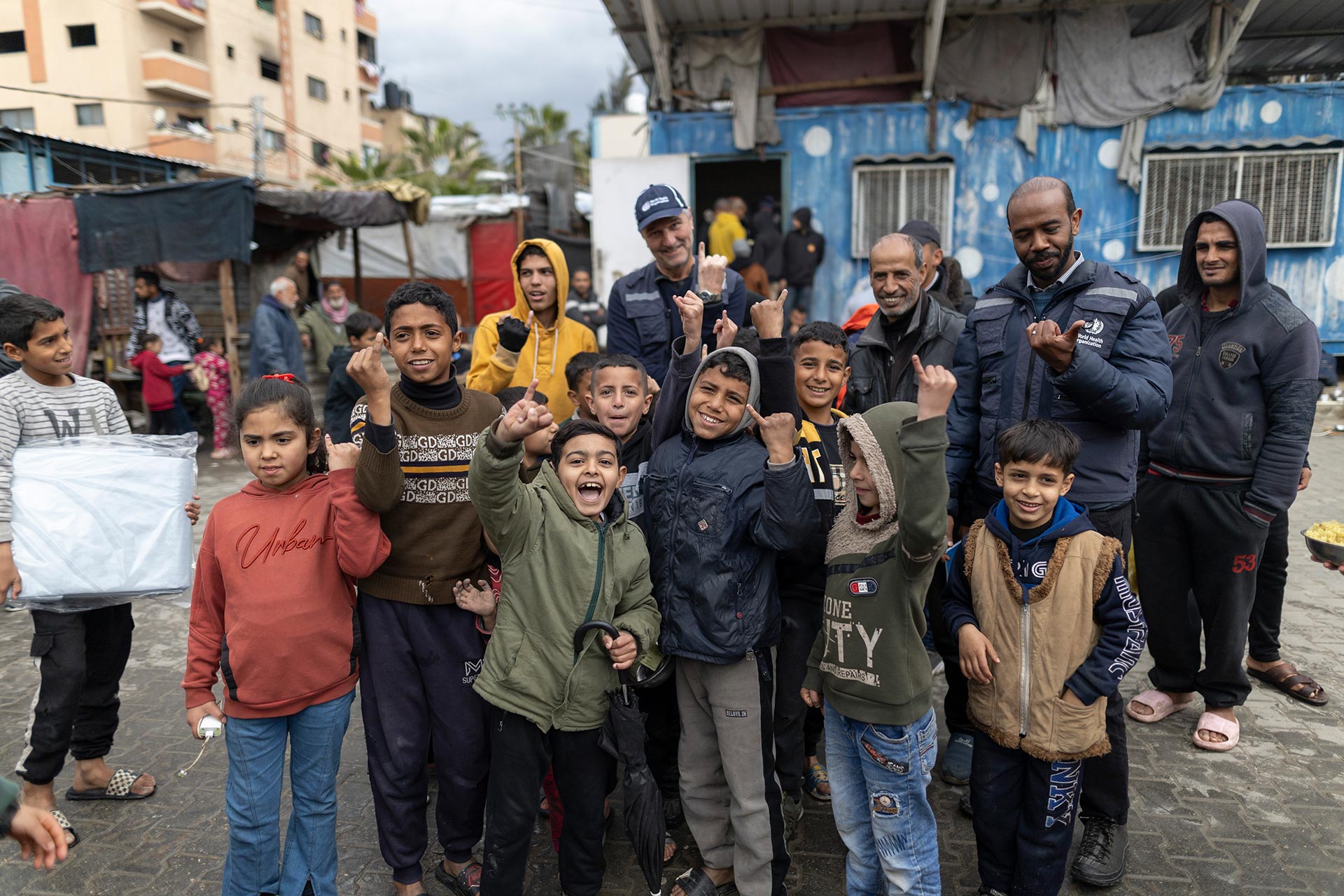
Seven ways WHO’s Eastern Mediterranean Region advanced polio eradication in 2025
In 2025, WHO’s polio programme in the Eastern Mediterranean Region advanced critical areas of eradication despite conflict, humanitarian crisis and funding pressures. From refining strategies in endemic…
Continue Reading
-

Nipah virus: Pakistan joins Asian countries by introducing checks for passengers after India outbreak
Pakistan has become the latest country to order enhanced screening of incoming travellers for signs of the deadly Nipah virus.
Thailand, Singapore Malaysia, Indonesia and Vietnam have already tightened airport screening. Authorities in Hong…
Continue Reading
-

Women with stroke history twice as likely to have another during or soon after pregnancy
Research Highlights:
- Female stroke survivors were more than twice as likely as their stroke-free counterparts to have another stroke while pregnant and in the six weeks after childbirth, according to an analysis of a large national…
Continue Reading
-
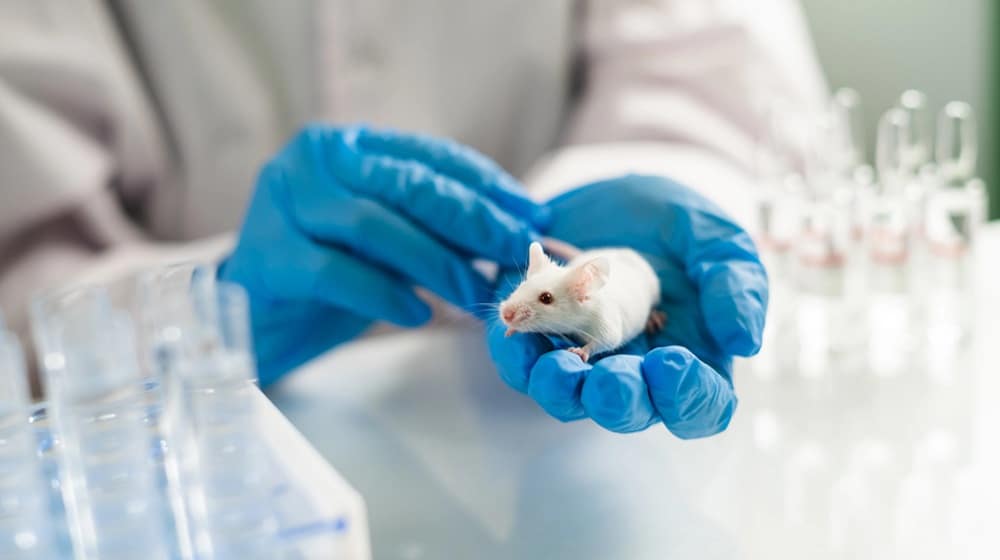
Scientists Successfully Eliminate Pancreatic Cancer in Mice for the First Time
A research team from Spain’s National Center for Oncological Research (CNIO), led by scientist Mariano Barbacid, has eliminated the most common and aggressive form of pancreatic cancer in mice for the first time.
The therapy was…
Continue Reading
-
Pakistan becomes latest Asian country to order checks for deadly Nipah virus – Al Arabiya English
- Pakistan becomes latest Asian country to order checks for deadly Nipah virus Al Arabiya English
- Why is India’s Nipah virus outbreak spooking the world? Al Jazeera
- Nipah virus: Some Asia airports screen passengers after outbreak in India BBC
Continue Reading
-
Pakistan is latest Asian country to step up checks for deadly Nipah virus – Arab News
- Pakistan is latest Asian country to step up checks for deadly Nipah virus Arab News
- Pakistan becomes latest Asian country to introduce checks for deadly Nipah virus Reuters
- Nipah virus: Some Asia airports screen passengers after outbreak in…
Continue Reading
-
No immediate Nipah virus threat in Sri Lanka: health official-Xinhua
COLOMBO, Jan. 29 (Xinhua) — A senior Sri Lankan health official said Thursday that there is no immediate threat to the country from the Nipah virus, which has been reported in parts of neighboring India.
Deputy Health Minister Hansaka…
Continue Reading
-
Pakistan becomes latest Asian country to introduce checks for deadly Nipah virus
LAHORE/HANOI, Jan 29 (Reuters) – Authorities in Pakistan have ordered enhanced screening of people entering the country for signs of infections of the deadly Nipah virus after India confirmed two cases, adding to the number of Asian countries…
Continue Reading
