Carmen Monge-Montero, Researcher and Global Cancer Advocate, shared a post on LinkedIn:
“Heading into holiday season = family gatherings = awkward cancer conversations?
I wanted to repost this article just before the holidays,…

Carmen Monge-Montero, Researcher and Global Cancer Advocate, shared a post on LinkedIn:
“Heading into holiday season = family gatherings = awkward cancer conversations?
I wanted to repost this article just before the holidays,…

Men’s brains appear to age more quickly than women’s, according to a recent study published in Proceedings of the National Academy of Sciences.
Brain ageing is a major risk factor for Alzheimer’s, the most common form of dementia, leading…
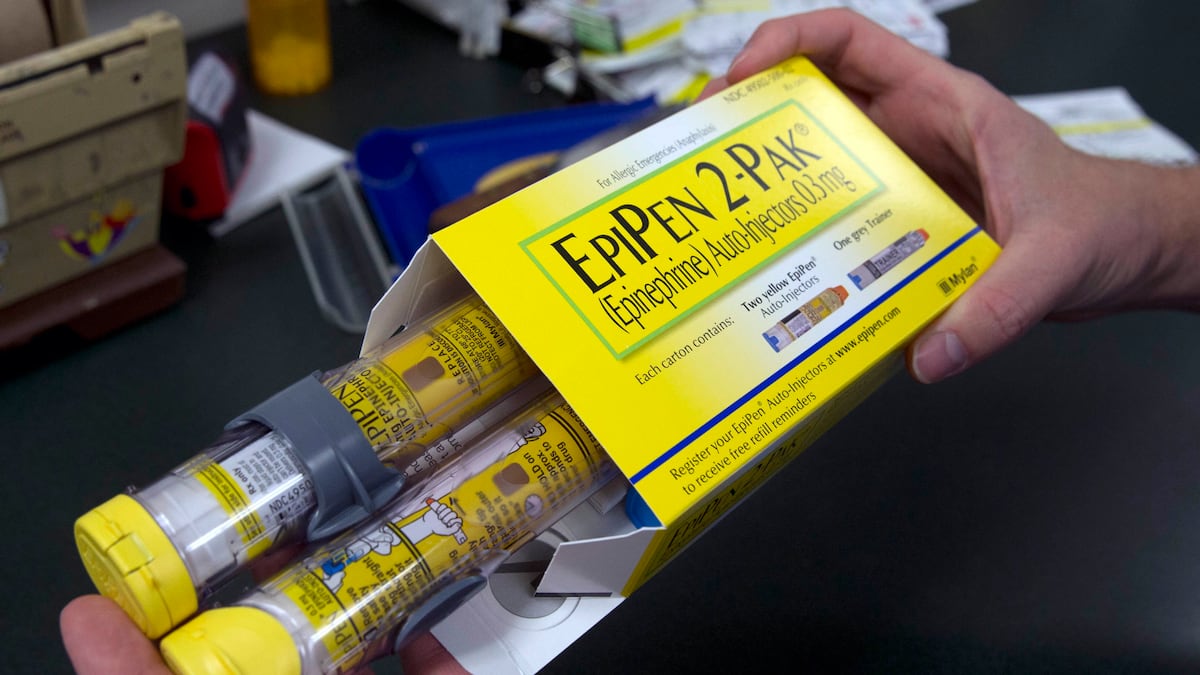
FILE – In this July 8, 2016, file photo, a pharmacist holds a package of EpiPens epinephrine auto-injector, a Mylan product, in Sacramento, Calif. Insurers will sometimes choose to cover a significantly more expensive drug or device, such as…
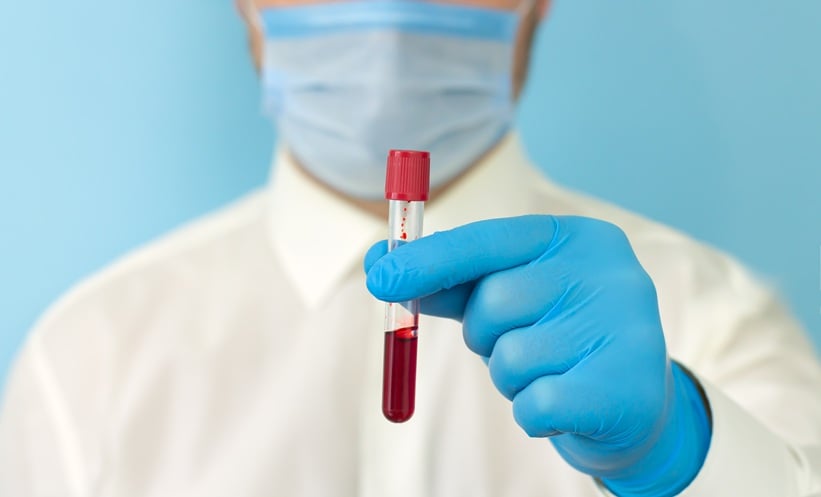
FT-IR microspectroscopy blood test detected a single circulating tumor cell (CTC) in one patient with lung cancer, cytospun raw blood sample.
Liquid biopsy is reshaping how clinicians think…

BREATH analysis may soon play a crucial role in asthma management, as new research highlights the potential of volatile organic compounds (VOCs) as non-invasive biomarkers for monitoring controlled asthma. A recent study examined the…
Goh, C. Y. & Ronco, C. Cardio-renal syndromes [J]. J. Ren. Care 36(Suppl 1), 9–17 (2010).
Ronco, C. et al. Cardiorenal syndrome [J]. J. Am. Coll. Cardiol. 52(19), 1527–1539 (2008).
Campisi, J. et al. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192 (2019).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer,…
Sweeteners such as aspartame, found in Equal packets, sucralose (Splenda), and sugar alcohols are widely promoted as healthier options than foods made with refined sugar (glucose). Many people turn to these alternatives hoping to reduce health…