On January 14, 2025, The Lancet Diabetes & Endocrinology published a Commission establishing an official definition and diagnostic criteria for
The central conceit of…

On January 14, 2025, The Lancet Diabetes & Endocrinology published a Commission establishing an official definition and diagnostic criteria for
The central conceit of…
![[Press release] MHRA approves lenacapavir for the prevention of sexually transmitted HIV-1 infection](https://afnnews.qaasid.com/wp-content/uploads/2025/10/1674900.jpg)
19 December 2025 — The Medicines and Healthcare products Regulatory Agency (MHRA)* has approved lenacapavir (Yeytuo) for the prevention of sexually transmitted HIV-1 infection in adults and adolescents.
The medicinal…

Richard Eby knew things were getting bad when the schools shut down.
Eby is a family medicine doctor based in Andrews, in West Texas. It’s a quiet town of 14,000, chock full of all-American touches — a Buffalo Wild Wings sits a few…

NEWS
![[Press release] WHO validates Brazil for eliminating mother-to-child transmission of HIV](https://afnnews.qaasid.com/wp-content/uploads/2025/12/15749213.jpeg)
18 December 2025 — The World Health Organization (WHO) has validated Brazil for the elimination of mother-to-child transmission of HIV, making it the most populous country in the Americas to achieve this historic milestone….

In early May 2025, NASA and partner satellites spotted a towering cloud of wildfire smoke rising over central Saskatchewan.
The huge plume, nicknamed a smoke monster, grew from intense fires burning in and around Narrow Hills Provincial Park.
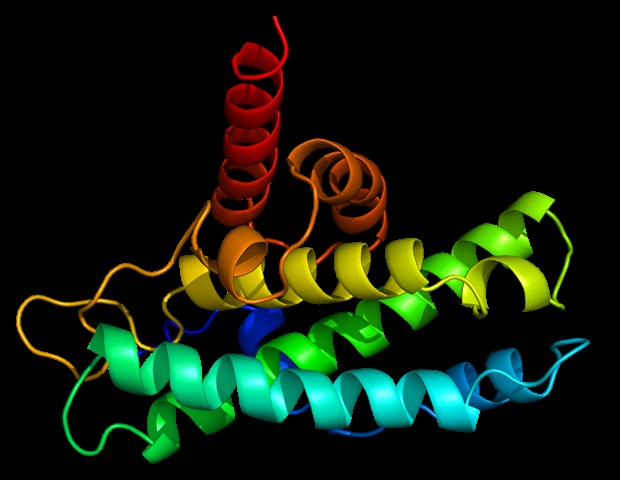
Scientists at the University of Colorado Anschutz have discovered that while brain neuron changes, including cell loss, may begin in early life, a drug long-approved for other conditions might be repurposed to slow this damage,…

This week, The Pharmaceutical Journal has been reflecting on the past year, considering how the pharmacy sector has grown and presenting the headline numbers from our coverage across pharmacy in 2025.
However, healthcare does not slow down as the…

Individuals who fall asleep quickly during overnight