WARSAW, Jan. 24 (Xinhua) — Poland’s largest population genomics initiative, “Genomics for Poland,” was launched on Saturday in Poznan, according to the Polish Press Agency.
Led by the Institute of Bioorganic Chemistry of the Polish Academy…
WARSAW, Jan. 24 (Xinhua) — Poland’s largest population genomics initiative, “Genomics for Poland,” was launched on Saturday in Poznan, according to the Polish Press Agency.
Led by the Institute of Bioorganic Chemistry of the Polish Academy…
A recent study led by the CDC reveals that routine vaccinations do not raise epilepsy risk in children, alleviating concerns over immunisation safety.
A comprehensive study led by the Centres for Disease Control and Prevention (CDC) in the…

Eating enough protein has become a buzzy, but worthwhile, nutrition goal. For starters, we need protein to function, explains Samantha Peterson, MS, RDN, functional medicine dietitian and founder of Simply Wellness. “It’s involved in nearly…

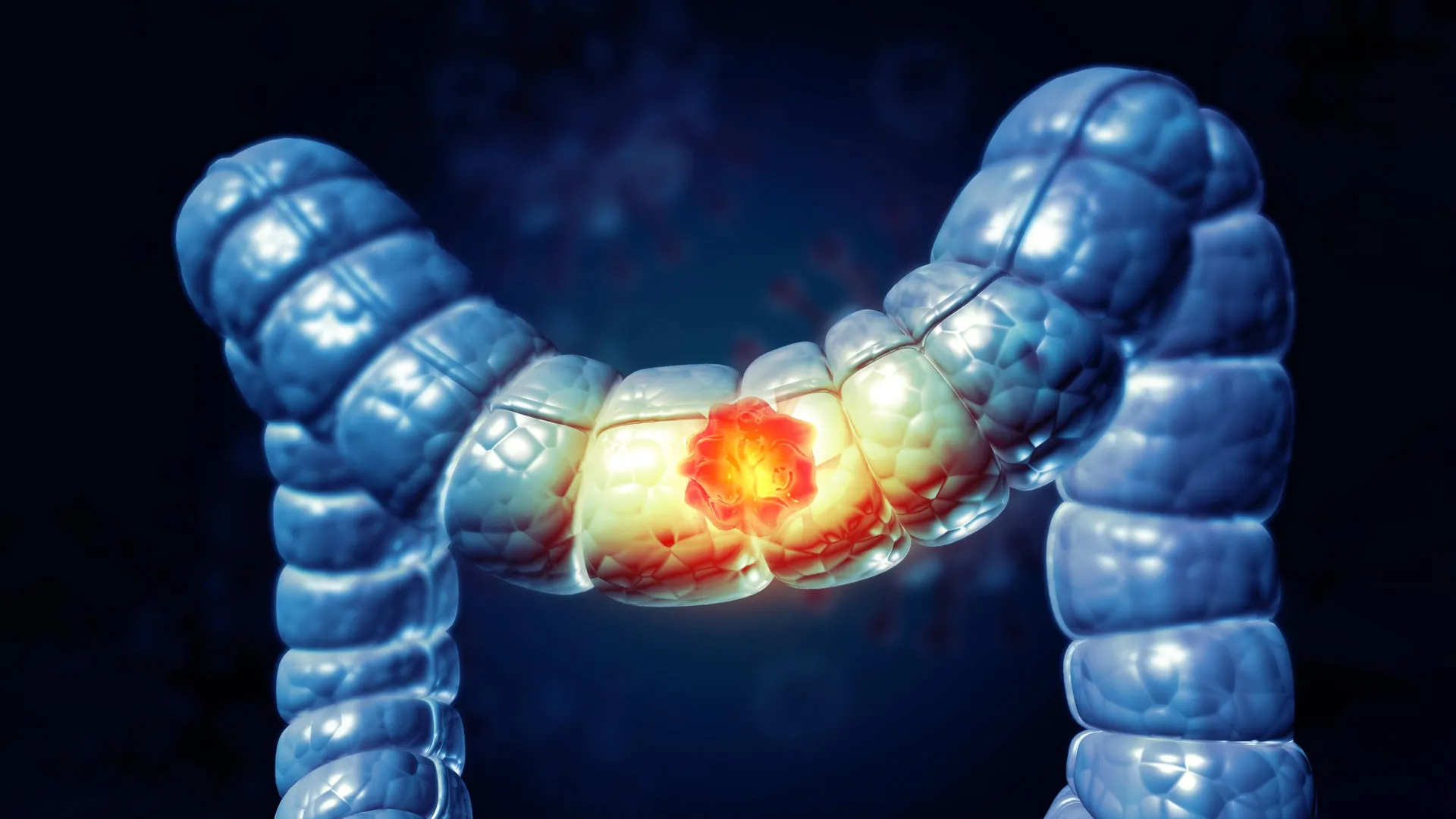
A new study suggests that long-term inflammation may physically change the colon in ways that promote early-onset colorectal cancer (CRC). Researchers found that chronic inflammation can increase the stiffness of colon tissue, potentially…

Children who show ADHD traits at age 10 are more likely to experience physical health problems and health-related disability by age 46, according to a study led by researchers at University College London (UCL) and the University of Liverpool.

Drinking too much coffee can overstimulate your nervous system, leading to jitters, anxiety, digestive upset, a racing heart, and sleep trouble—especially above about 400 mg of caffeine per day.
If you overdo it, stop caffeine,…