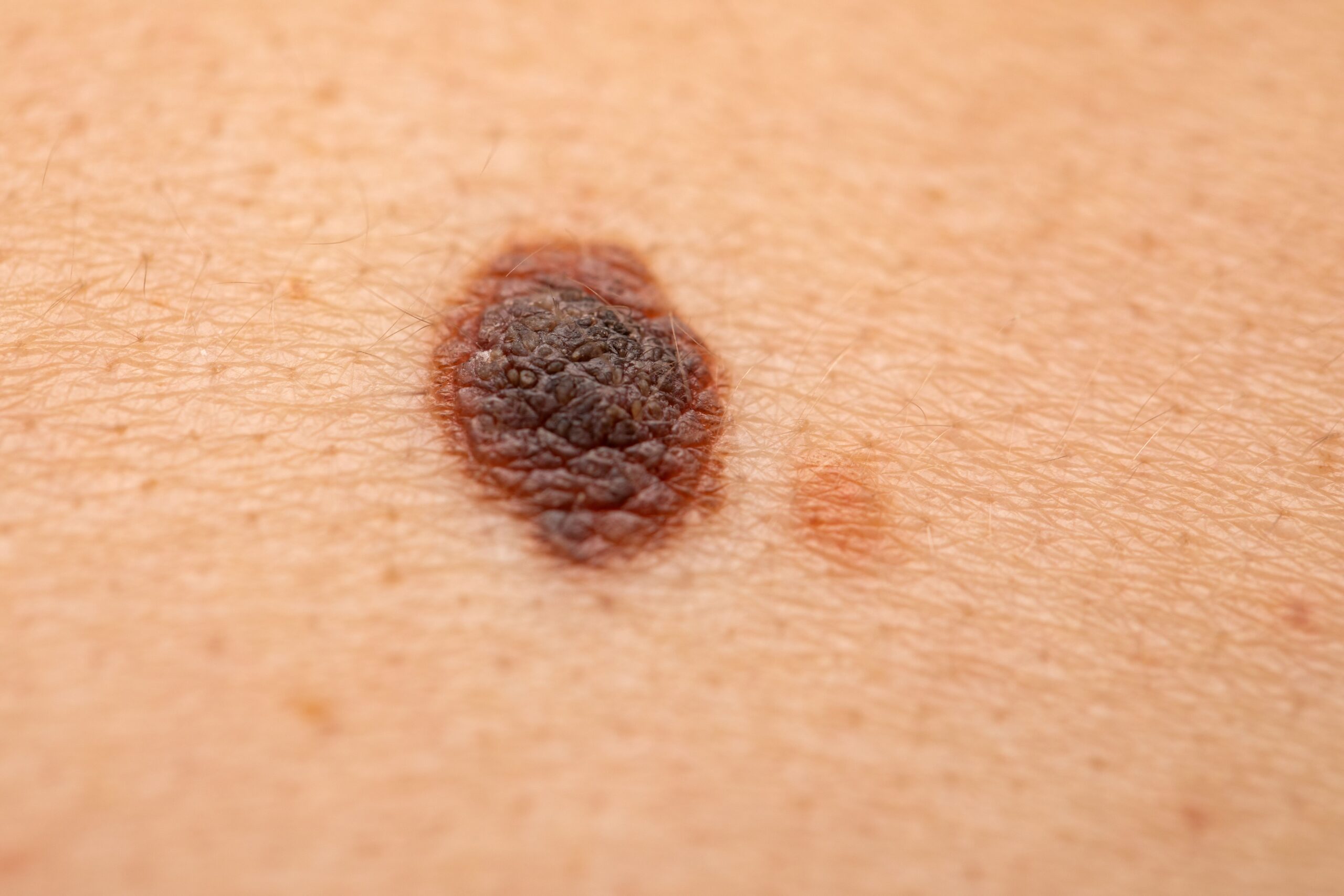
Melanoma is one of the cancers with declining a mortality rate in the United States, according to the American Cancer Society’s annual report released in January 2025. The
Midlife influenza vaccination did not raise the risk of Parkinson’s disease in a large…