Category: 6. Health
-
Study reveals the hidden toll of sudden cardiac death in type 1 and type 2 diabetes
A Danish nationwide study shows that people with type 1 and type 2 diabetes face much higher rates of sudden cardiac death and lose years of life as a result, highlighting the urgent need to identify and protect those at greatest…
Continue Reading
-
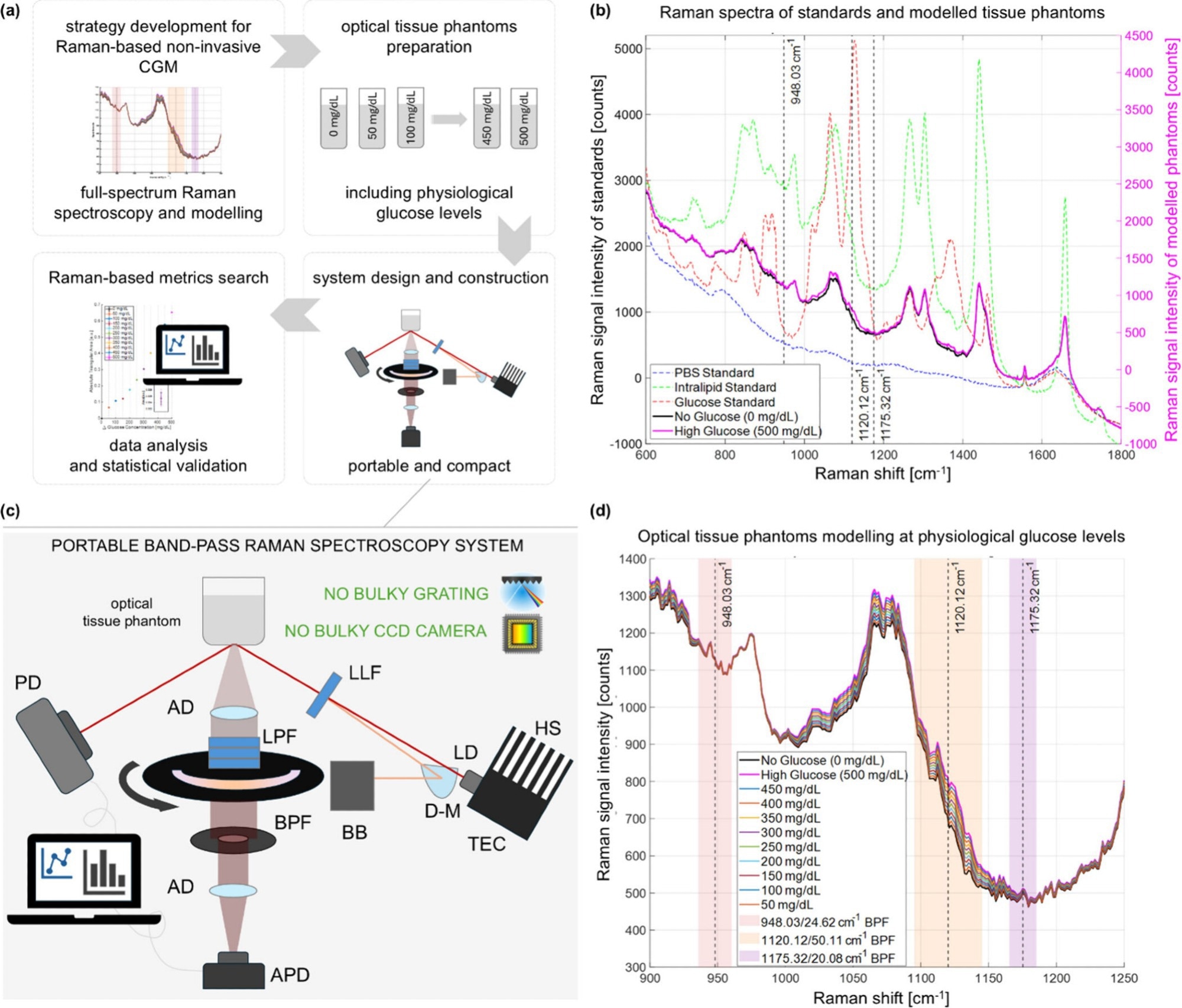
Noninvasive Raman device tracks blood glucose accurately in under a minute
A shoebox-sized optical device reads glucose directly through the skin in seconds, offering a promising step toward truly noninvasive, point-of-care glucose monitoring.
Pipeline for the development of compact BRS-based CGM. (a)…
Continue Reading
-

Young Australians are staying on antidepressants for longer than ever
A nationwide analysis of prescription data reveals that young Australians are staying on antidepressants for longer than ever, highlighting growing gaps between clinical guidelines and real-world practice.
Increasing Prevalence of…
Continue Reading
-

Virtual diet and exercise program shows promise in reducing lymphoma treatment side effects
Patients undergoing treatment for lymphoma often experience adverse side effects that can be so severe that they stop or slow treatment. But a new study shows that a virtual program focusing on diet and exercise is a feasible…
Continue Reading
-

Singapore woman had daughter through IVF, ensuring baby would not inherit her genetic condition
December 8, 2025
SINGAPORE – She was only 12 when she was diagnosed with familial adenomatous polyposis (FAP), an inherited condition that causes hundreds or thousands of polyps to form in the colon and rectum.
And when she turned 15, homemaker…
Continue Reading
-

Winter is coming for your sperm? Doctor shares 6 simple steps to boost male fertility in colder months
If you thought winter only messes with your mood, dry skin and social life, think again as it may also be quietly influencing male fertility. Not because…
Continue Reading
-

Kiwifruit intake boosts vitamin C in skin and supports dermal structure
By directly measuring vitamin C inside human skin, researchers show that diet can boost skin vitamin C content and influence skin structure, while also revealing clear limits to its effects on collagen formation and UV protection.
Continue Reading
![[Exclusive] Singapore looks to add hereditary cancers, kidney diseases in expanded genetic testing](https://afnnews.qaasid.com/wp-content/uploads/2025/12/1763115282444.jpeg)
[Exclusive] Singapore looks to add hereditary cancers, kidney diseases in expanded genetic testing
[Exclusive] Singapore looks to add hereditary cancers, kidney diseases in expanded genetic testing | Healthcare IT News …
Continue Reading

Disease prevention has taken a hit from U.S. vaccine advisers, experts say
LOS ANGELES –U.S. vaccine advisers’ decision to scrap longstanding guidance on hepatitis B shots will expose more children to the harmful virus and may signal how…
Continue Reading