Parkinson’s disease is often considered an illness of old age, but now early signs are seen even in young adults, in their 20s, 30s, and 40s. Before the condition fully develops, it gives off subtle clues that many overlook or dismiss as stress…
Category: 6. Health
-

Rabid jackal detected in Dalton, Galilee; exposed person referred for treatment
The Ministry of Health announced that a rabid jackal was detected in the settlement of Dalton in the Upper Galilee. A person exposed to the jackal was referred for preventive rabies treatment. The ministry requests anyone who had contact or whose…
Continue Reading
-
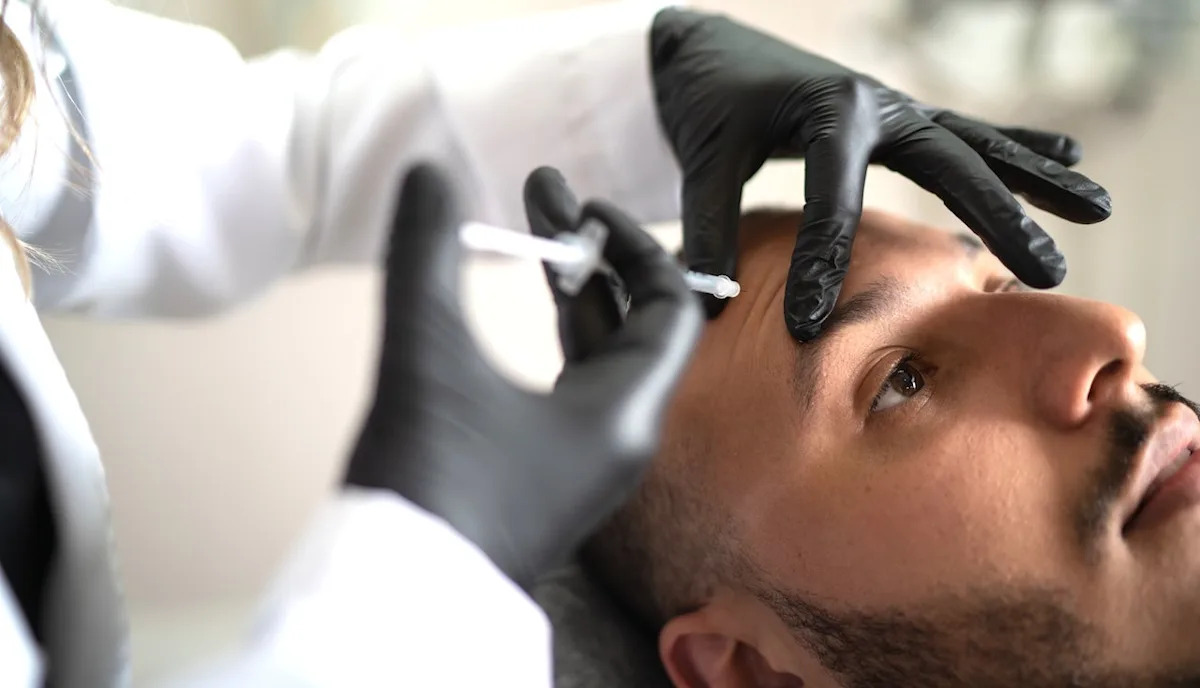
Cosmetic fillers can cause deadly complication, experts warn — but new tech exposes it
Each year, more than 5 million cosmetic filler procedures are performed in the U.S. — but these injectables can potentially block key blood vessels, putting patients at risk for serious harm.
In a study presented this week at the annual meeting…
Continue Reading
-

Constipation is not the first sign of fibre deficiency, it is… | Health News
From weight management to supporting bowel movement and digestion, fibre does it all. But did you know that constipation is not the very first sign of a lack of fibre within the body? According to Dr Leena Saju, Group Manager, Clinical Nutrition,…
Continue Reading
-
50 children dead in Somalia amid diphtheria outbreak-Xinhua
MOGADISHU, Dec. 7 (Xinhua) — Somalia’s Ministry of Health and Human Services on Sunday confirmed that a new diphtheria outbreak has killed 50 children and infected about 1,000 others nationwide.
In a statement, the ministry said children…
Continue Reading
-
50 children dead in Somalia amid diphtheria outbreak-Xinhua
MOGADISHU, Dec. 7 (Xinhua) — Somalia’s Ministry of Health and Human Services on Sunday confirmed that a new diphtheria outbreak has killed 50 children and infected about 1,000 others nationwide.
In a statement, the ministry said children…
Continue Reading
-

Kim Kardashian’s Doctor Finds Holes in Her Brain, Signs of “Low Activity”
Reality TV star Kim Kardashian is still struggling to pass her bar exam. Last month, she revealed that she’d failed the test, a major setback in her journey to become an attorney, and she’s even accused ChatGPT of making her “fail tests…
Continue Reading
-

How higher fitness levels cut the risk of bladder and kidney cancer, according to a 22-year study |
According to the European Medical Journal, adults with higher cardiorespiratory fitness may have a significantly lower risk of developing urinary tract cancers, including bladder and kidney cancer. These findings come from the second Trøndelag…
Continue Reading
-

11 Healthy Foods Women Over 50 Should Be Eating, According to Dietitians
There’s no doubt that getting older is a privilege. However, aging comes with a plethora of changes, especially in terms of nutritional needs. Think about it: Diet plays a major role in overall health and longevity, and after 50, your heart,…
Continue Reading
-

What You Need to Know
- The carnivore diet includes only animal-based foods, like beef, seafood and eggs.
- Proponents say it can get to the root cause of illness and relieve symptoms.
- This diet is super restrictive, lacks many essential nutrients and is not sustainable…
Continue Reading
