The Namibia Ministry of Health reported six cholera cases, with three confirmed, and no deaths from Grootfontein District, Otjozondjupa Region on November 24.
This is the second cholera outbreak reported in 2025.
This year, a cumulative of 24 cases…

The Namibia Ministry of Health reported six cholera cases, with three confirmed, and no deaths from Grootfontein District, Otjozondjupa Region on November 24.
This is the second cholera outbreak reported in 2025.
This year, a cumulative of 24 cases…

Flu cases in Scotland are expected to “spike” in the coming weeks, the health secretary has warned.
Neil Gray MSP said rates of the illness are following a similar trend…
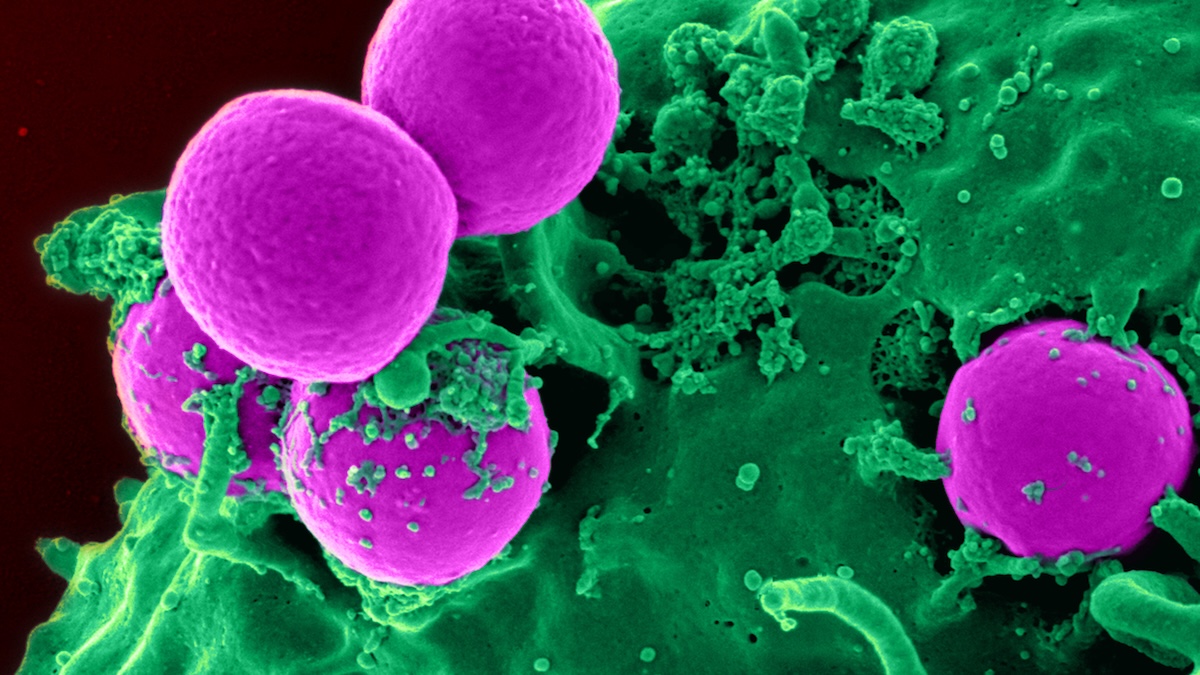
Dangerous bacteria and other disease-causing microbes are rapidly evolving ways to defy our best antibiotic medications, a phenomenon known as antimicrobial resistance. Humans are inadvertently contributing by overexposing pathogens to our…
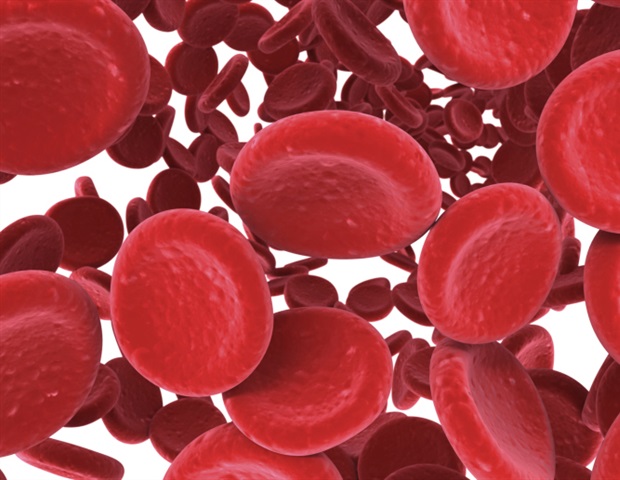
Treatment with intravenous (IV) iron significantly improved survival and increased hemoglobin levels in patients with iron-deficiency anemia who were hospitalized for an acute bacterial infection, according to an analysis of data…
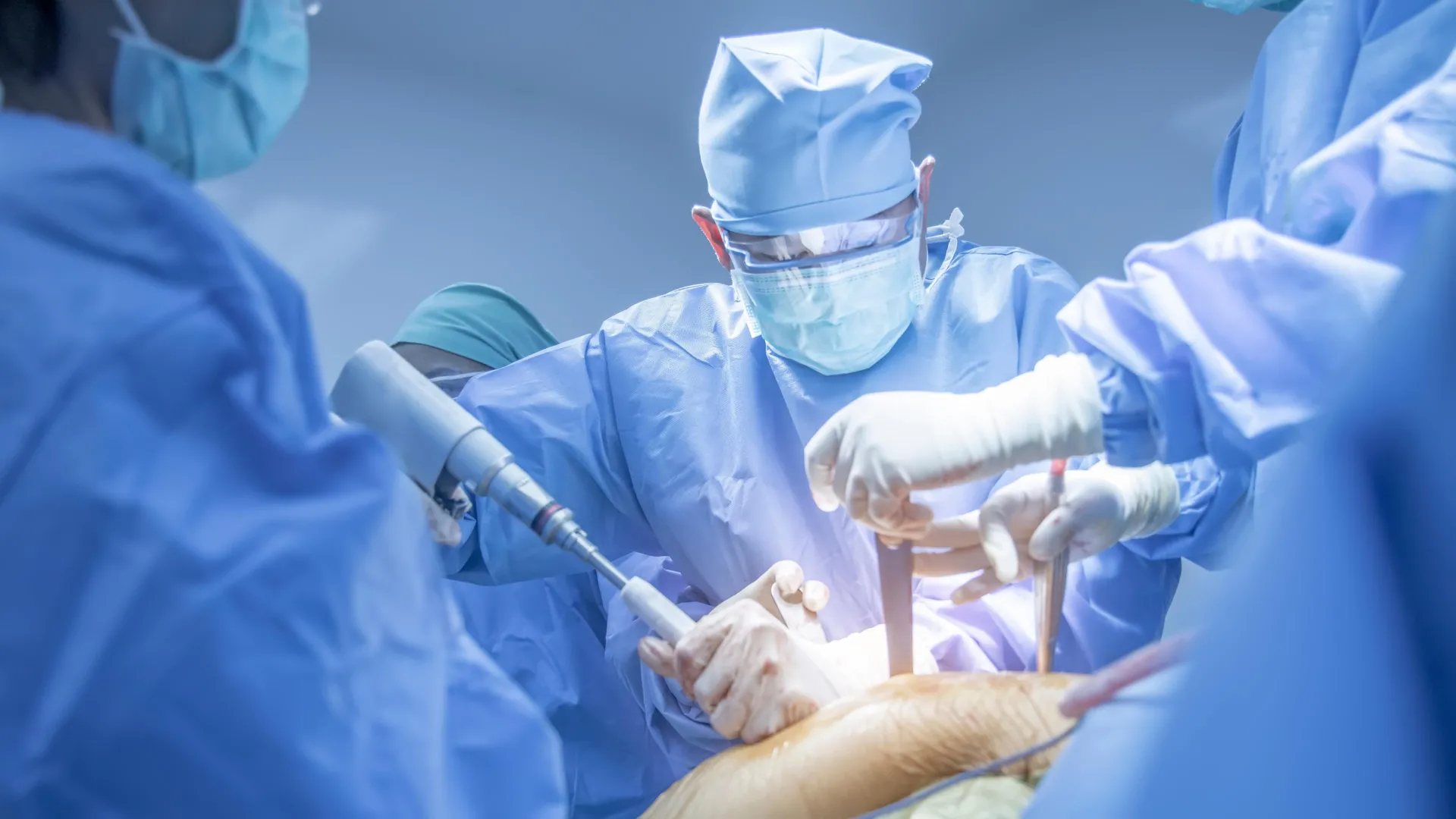
A recent study in the Journal of Hepatology describes the first successful auxiliary liver xenotransplant from a genetically engineered pig into a living human. The recipient survived for 171 days, providing early evidence that modified porcine…

Taking the sickle cell drug hydroxyurea during or shortly before pregnancy does not appear to cause specific issues in newborns, according to the first prospective study of pregnancies involving hydroxyurea exposure.
Since there…
Hajarizadeh, B., Grebely, J. & Dore, G. J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol 10, 553–562 (2013).
Manns, M. P. et al. Hepatitis C virus infection. Nat. Rev. Dis. Prim. 3, 17006 (2017).

Welcome to NeurologyLive® Brain Games! This weekly quiz series, which goes live every Sunday morning, will feature questions on a variety of clinical and historical neurology topics, written by physicians, clinicians, and experts in the fields…