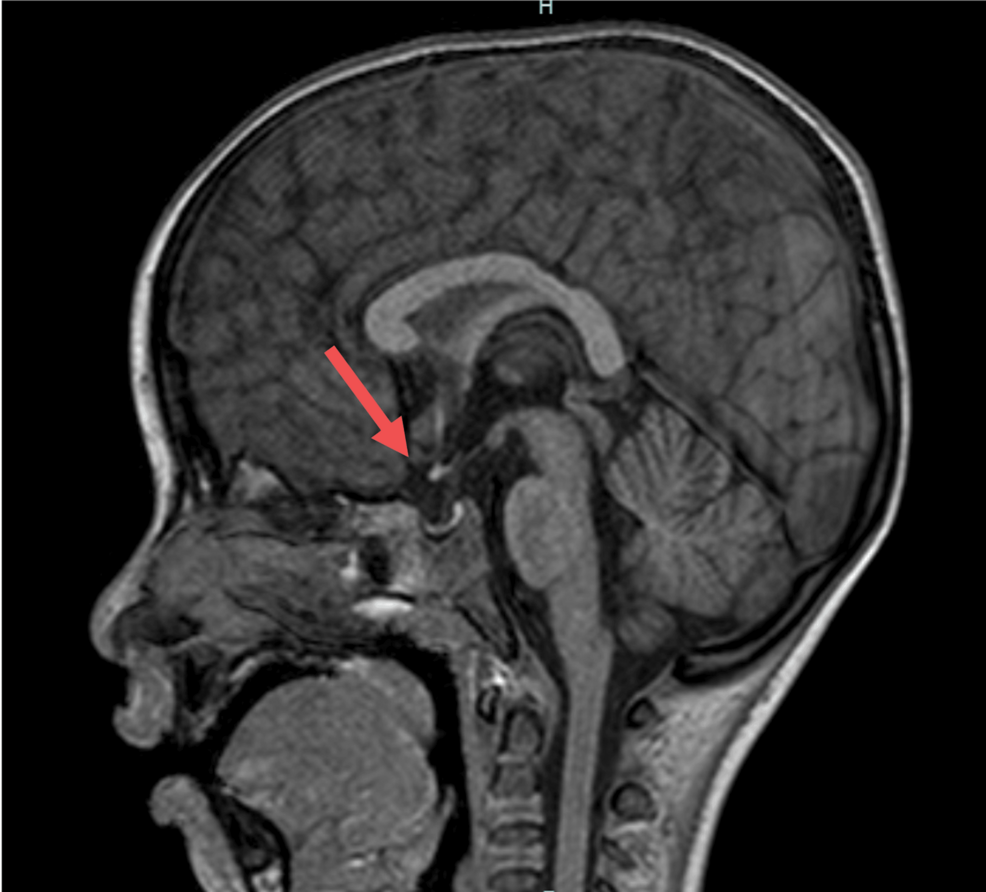
- CDC Panel Ends Call for All Newborns to Get Hepatitis B Vaccine Bloomberg.com
- Kennedy’s vaccine advisory committee delays vote on hepatitis B shots for newborns AP News
- CDC advisers vote to overturn decades-long policy on hepatitis B vaccine…
Category: 6. Health
-
CDC Panel Ends Call for All Newborns to Get Hepatitis B Vaccine – Bloomberg.com
-

7 foods to boost your immunity in cold weather
As the temperature drops and there’s a chill in the air, in winter, we often tend to gravitate towards what keeps us warm. Struggling with the dip in temperature, our bodies face a twin challenge: the cold itself, which can suppress immune…
Continue Reading
-

5 Endocrinology Headlines You Missed in November 2025
While largely a quiet month for endocrinology, November 2025 was not without its important updates. There were no significant approvals from the
US Food and Drug Administration , but glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have…Continue Reading
-
Trump signs memo to align US child vaccines with certain other countries – Reuters
- Trump signs memo to align US child vaccines with certain other countries Reuters
- Kennedy’s vaccine advisory committee delays vote on hepatitis B shots for newborns AP News
- CDC advisers vote to overturn decades-long policy on hepatitis B vaccine…
Continue Reading
-

RFK Jr.’s panel ends Hepatitis B vaccine recommendation for newborns: What is it and why was it required? |
On December 5th, Health and Human Services Secretary Robert F. Kennedy Jr.’s vaccine advisory panel voted to no longer universally recommend the first dose of Hepatitis B vaccine for newborns within 24 hours of…
Continue Reading
-
US vaccine committee scraps recommendation for hepatitis B shot in all newborns – Reuters
- US vaccine committee scraps recommendation for hepatitis B shot in all newborns Reuters
- Kennedy’s vaccine advisory committee delays vote on hepatitis B shots for newborns AP News
- CDC advisers vote to overturn decades-long policy on hepatitis B…
Continue Reading
-

Volcanic eruptions may have brought Black Death to Europe – Newspaper
PARIS: Previously unknown volcanic eruptions may have kicked off an unlikely series of events that brought the Black Death — the most devastating pandemic in human history — to the shores of medieval Europe, new research has revealed.
The…
Continue Reading
-

How bioprospecting revealed a scorpion venom that kills breast cancer cells
Researchers at the University of São Paulo’s Ribeirão Preto School of Pharmaceutical Sciences (FCFRP-USP) in Brazil have found a molecule in the venom of Brotheas amazonicus, a species of scorpion native to the Amazon, which appears to attack…
Continue Reading
-
Federal Panel Votes to Remove Universal Newborn Hepatitis B Vaccine Recommendation
The Advisory Committee on Immunization Practices (ACIP) — a panel that shapes policy around vaccinations on behalf of the Centers for Disease Control and Prevention (CDC) — voted today to reverse a long-standing recommendation for infants to…
Continue Reading