By Cassandra Calabrese, DO
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
At the end of 2023, the U.S….

By Cassandra Calabrese, DO
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
At the end of 2023, the U.S….
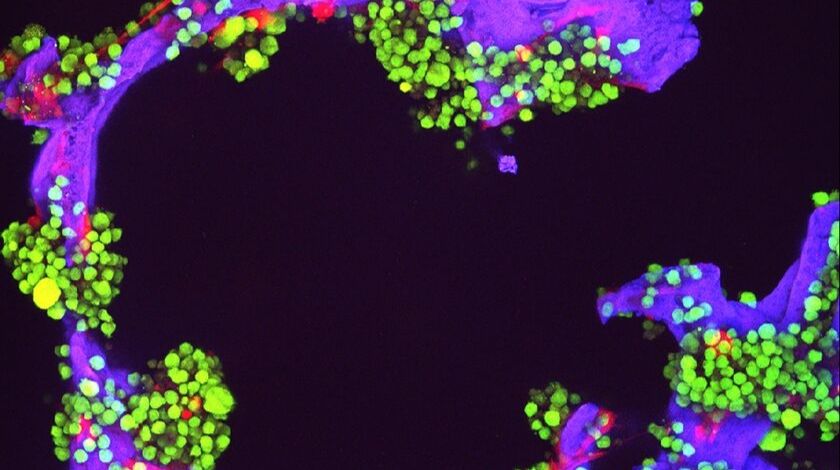
A study has unearthed potential reasons for sex differences that are observed in the clinical presentation of patients with newly diagnosed multiple myeloma.
Researchers at the University of Alabama at Birmingham (AL, USA) have…

Xinhuan Wang, Ji Li, Zhihao Li, Yuan Chen, Yubei Li, Xiaodong Li,* Yang Han*
Department of Dermatology, Affiliated Central Hospital of Shenyang Medical College, Shenyang, Liaoning, People’s Republic of China
Correspondence: Xiaodong Li,…
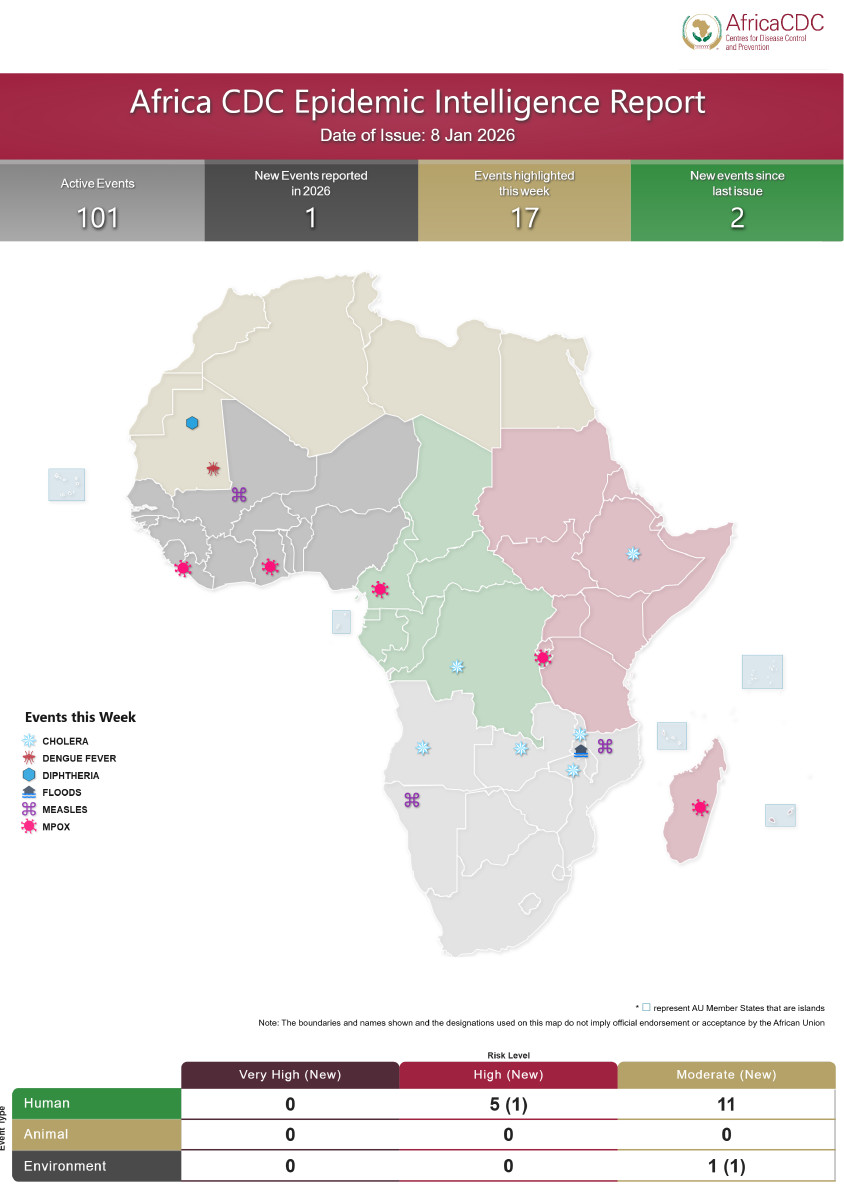
…

For more than a century, scientists and food companies have been looking for ways to replicate the taste of sugar without its health drawbacks. From early sweeteners like saccharin in the 19th century to modern alternatives such as stevia and…
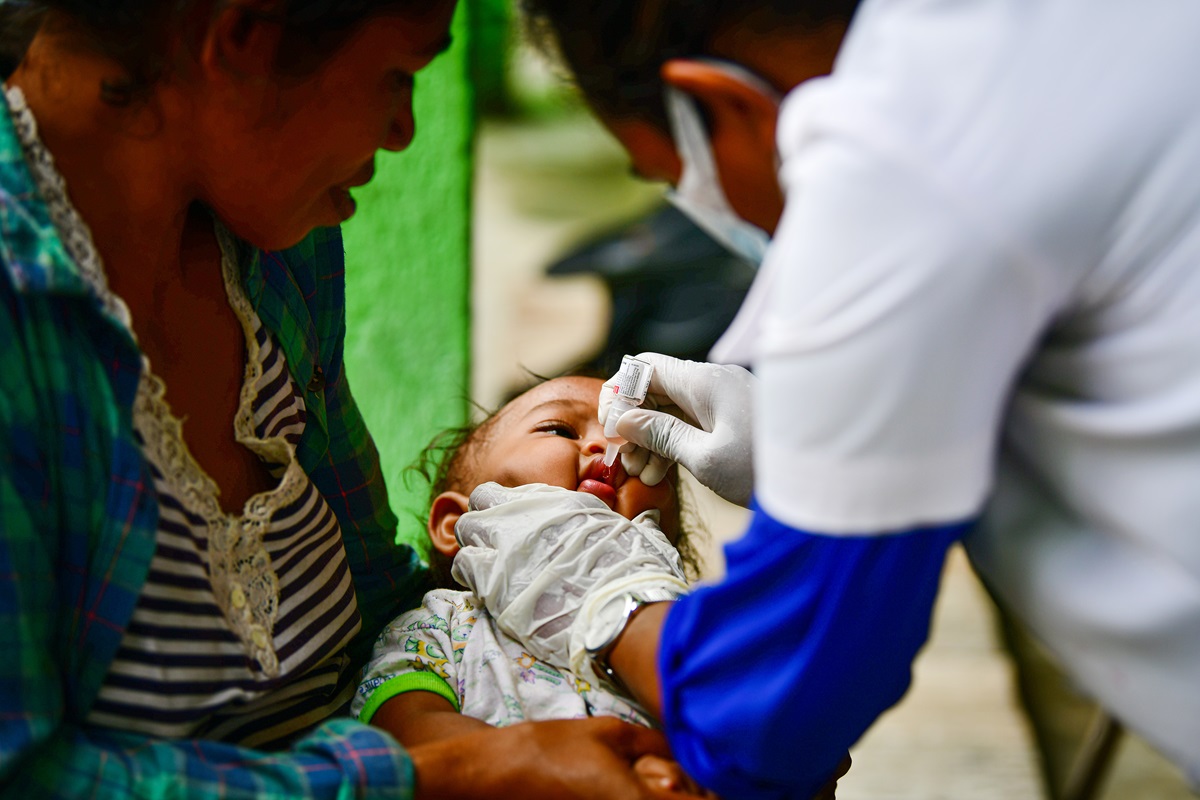
Fifteen years after recording its last case of wild poliovirus, the WHO South-East Asia Region with a quarter of the world’s population, continues to sustain its polio-free status while harnessing innovations and lessons from the polio…
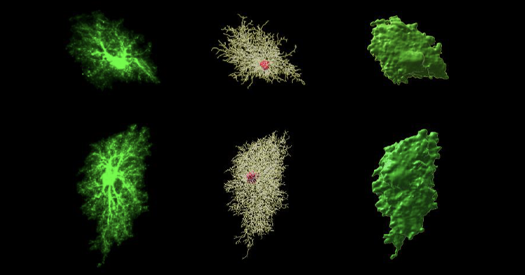
Cognitive aging: Several new papers address interactions between autism and dementia, including comorbidity and influential factors. A large study of Medicare and Medicaid records found that more autistic adults develop senile…
Tan, J. K. & Bhate, K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 172, 3–12 (2015).
Goulden, V., Stables, G. I. & Cunliffe, W. J. Prevalence of facial acne in adults….
CANBERRA, Jan. 13 (Xinhua) — Blocking a key cellular enzyme, an approach thought to protect against fatty liver disease, may instead increase the risk of chronic liver damage and cancer as people age, Australian researchers warned on Tuesday.
…