SINGAPORE, March 5 (Xinhua) — Singapore will introduce an artificial intelligence (AI) assessment tool in early 2027 to help identify individuals at high risk for cardiovascular disease, the Ministry of Health said Thursday.
Developed by…
SINGAPORE, March 5 (Xinhua) — Singapore will introduce an artificial intelligence (AI) assessment tool in early 2027 to help identify individuals at high risk for cardiovascular disease, the Ministry of Health said Thursday.
Developed by…
Jebari-Benslaiman, S. et al. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23063346 (2022).
Bergstrom, G. et al. Prevalence of subclinical coronary Artery atherosclerosis in the general population….
Attention-deficit/hyperactivity disorder (ADHD) is often talked about as if it were a single condition. But anyone who works with children with ADHD-or raises one-knows that symptoms can look very different from one child to…
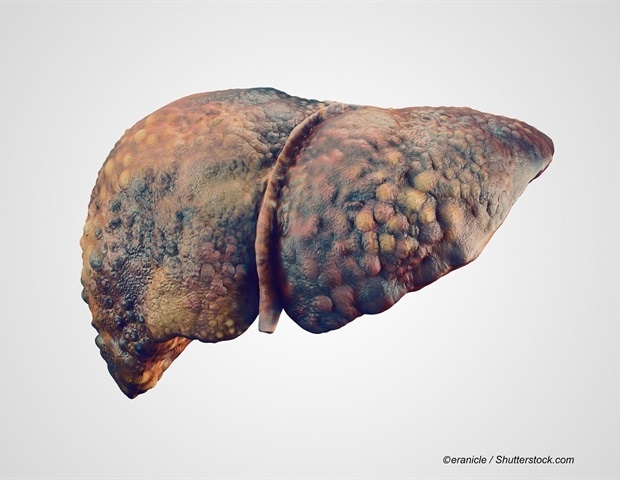
Researchers at the Johns Hopkins Kimmel Cancer Center report that an artificial intelligence (AI)-based liquid biopsy test using genome-wide cell-free DNA (cfDNA) fragmentation patterns and repeat landscapes can detect early liver…

Gov.UK New targeted trial in turkeys will test vaccine efficacy as part of fight to protect wild and captive birds. Highly pathogenic avian influenza (HPAI) vaccine trials have started…

Nearly a year and a half after infection, researchers examined whether long COVID leaves measurable traces of inflammation or neuronal damage in the blood and their findings challenge assumptions about persistent immune activation.
A meta-analysis published in eClinicalMedicine looks at weight regain trajectories after stopping GLP-1 receptor…

The HIll Jay Bhattacharya, the recently appointed acting director of the Centers for Disease Control and Prevention (CDC), on Monday urged families to consider vaccinating against measles as
Stiff knees, sore hips, and persistent joint pain are often brushed off as normal signs of aging. But osteoarthritis, the most common joint disease worldwide, is not being treated in line with what research actually shows. Experts say the biggest…