Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder with an unclear etiology. It is characterized by progressive joint inflammation, pain, and deformities. In advanced stages, these manifestations can lead to functional…

Rheumatoid arthritis (RA) is a chronic autoimmune disorder with an unclear etiology. It is characterized by progressive joint inflammation, pain, and deformities. In advanced stages, these manifestations can lead to functional…

In sub-Saharan Africa (SSA), family planning is crucial for public health and socioeconomic development, as high fertility rates and rapid population growth lead to increased maternal and child mortality, strain on the healthcare…
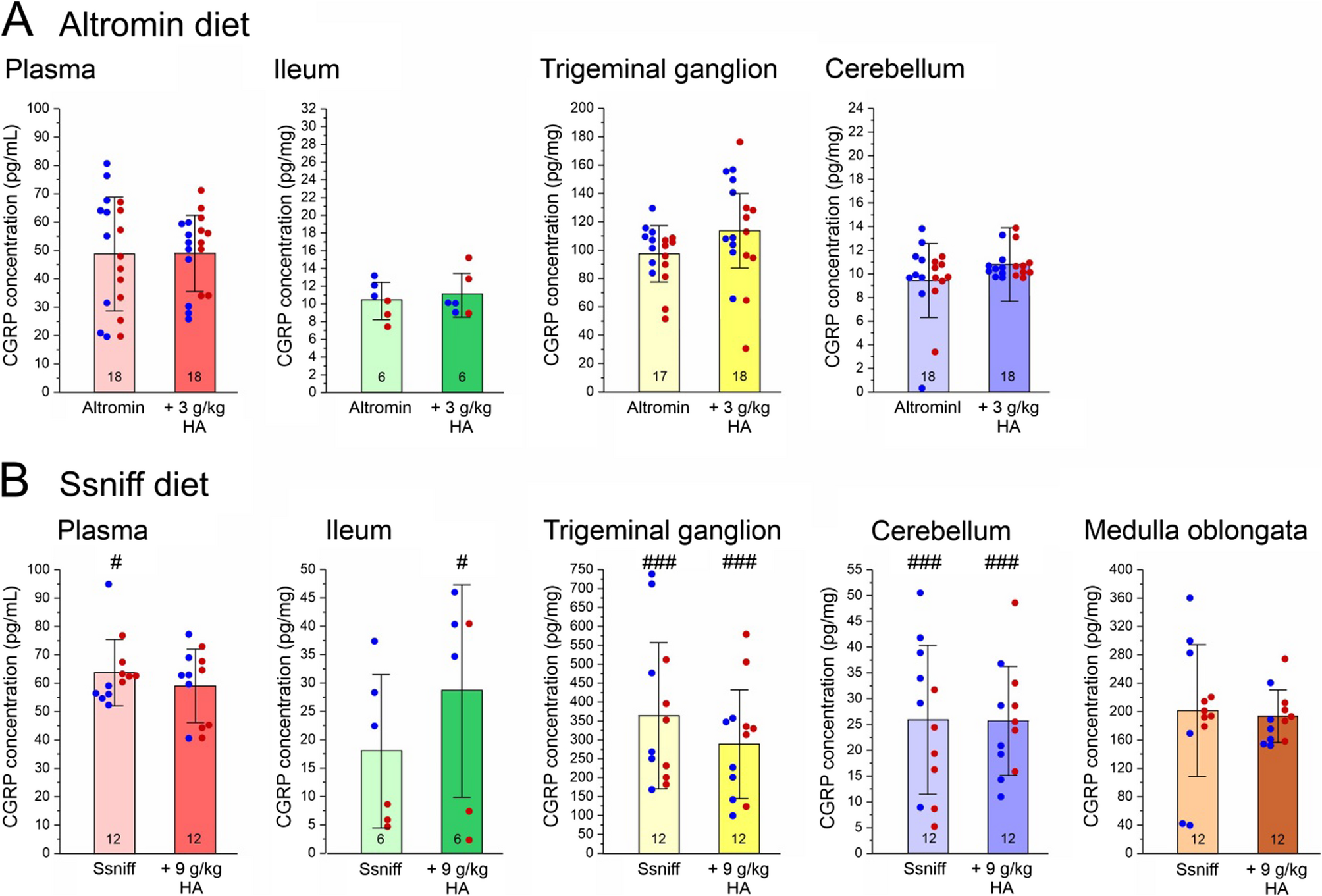
For this study, 60 mice of both sexes at equal numbers were used. Details concerning the condition of animals such as changes in body weight have been reported in our recently published paper [3]. Experimental groups of…


Globally, liver cancer ranks as the sixth most common cancer and the third leading cause of cancer-related deaths.1 HCC is the most common type of liver cancer, which accounts for 90% of all the cases.2 Although the incidence and…
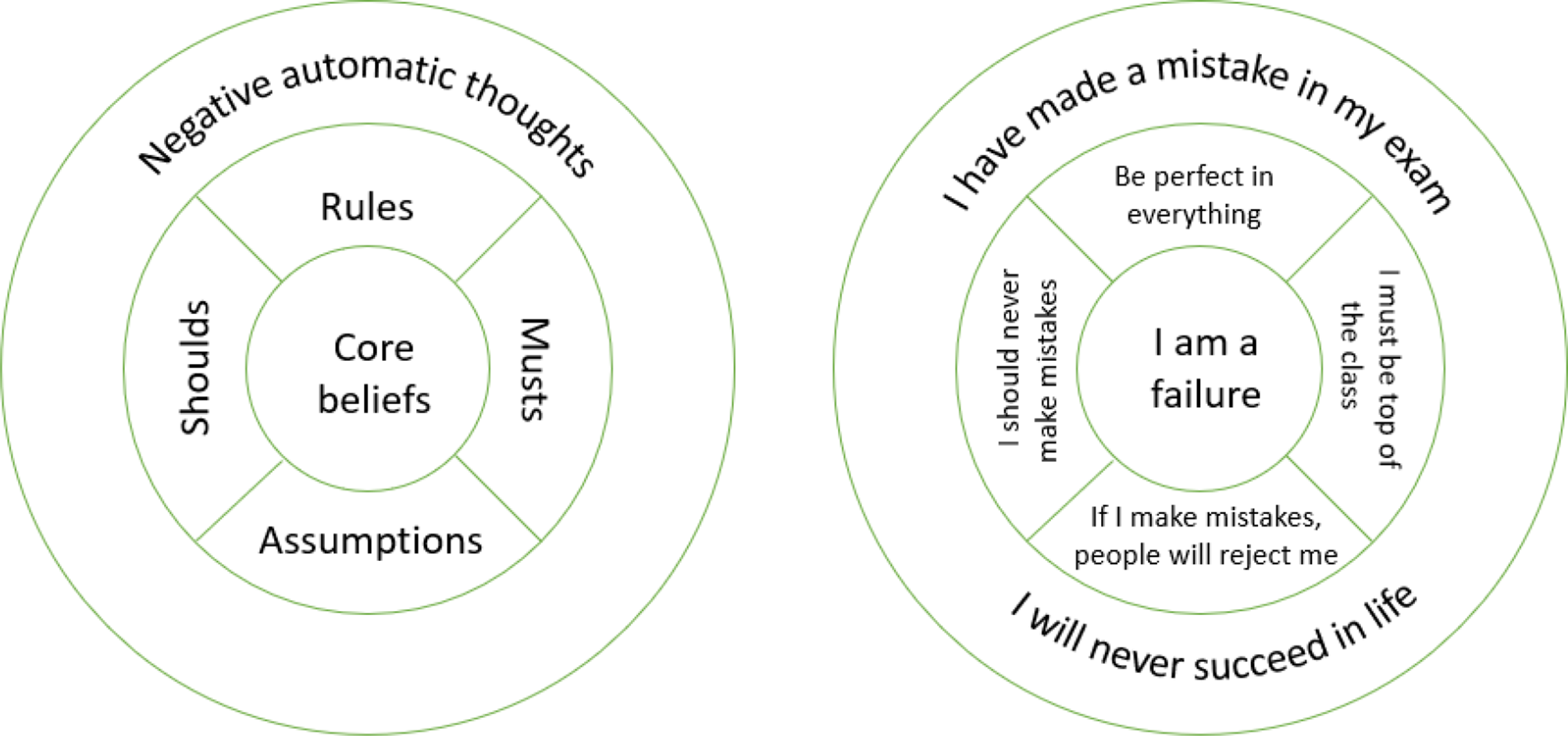
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders [Internet]. 2013 [cited 2024 Jun 1]. Available from: https://doi.org/10.1176/appi.books.9780890425596
Sanchez-Cerezo J, Nagularaj L, Gledhill J, Nicholls D….
It may have begun with the virus becoming active in the local mosquito population, prompted by heavy rains followed by warm weather — ideal conditions for the mosquitoes responsible for spreading the virus. Already present in the…

Robotic-assisted thoracic surgery (RATS) has become an established approach for managing a range of thoracic pathologies, particularly in lobectomies and other common interventions. With its enhanced visualization, precision, and…

Prof. Takashi Hiiragi (Hubrecht Institute), Dr. Robert Prevedel (European Molecular Biology Laboratory), and Dr. Lukas Krainer (Prospective Instruments) have launched an interdisciplinary collaboration to develop a novel intravital…