What is your research about?
I work mainly on inherited bleeding disorders, which include well-known ones such as haemophilia and lesser-known ones like von Willebrand disease. What they have in common is that they make people more prone to…

I work mainly on inherited bleeding disorders, which include well-known ones such as haemophilia and lesser-known ones like von Willebrand disease. What they have in common is that they make people more prone to…

Few primary care practices are designed for the timely detection of Alzheimer’s disease and related dementias. The limited time that primary care clinicians are able to spend with patients, the need to focus on the health problems…
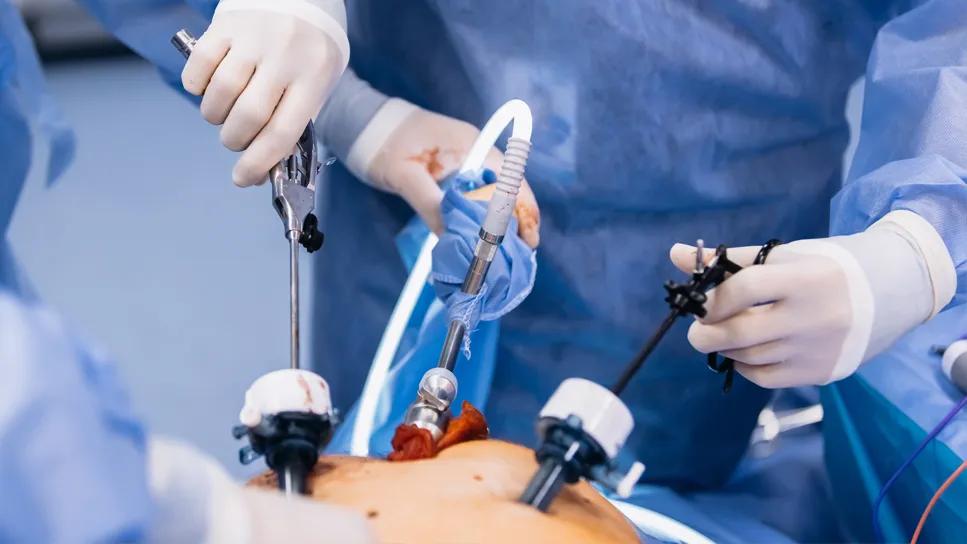
Adrenalectomy is a complex, low-volume procedure — only around 7,000 are performed annually in the U.S. compared to about a million gallbladder surgeries.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our…

Samantha Vaughan went to see her GP after her symptoms of long Covid didn’t seem to be improving.
She was referred for ENT (ear, nose and throat) examinations, along with a chest X-ray. “I was feeling breathless and fatigued and although it…
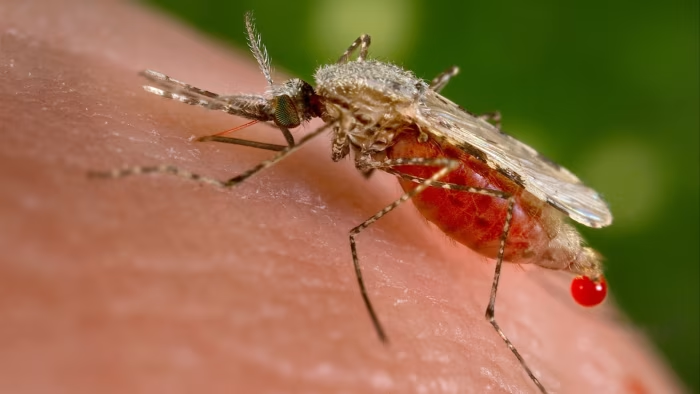
An experimental drug was effective against malaria in a new study, potentially offering a much-needed buffer against the rising threat of drug resistance.
The drug, known as GanLum, is made by Swiss pharmaceutical giant Novartis. In a…

When we talk about medications like Ozempic and Wegovy, the conversation often centres on weight loss, blood sugar control and body-image transformations….
Juba – South Sudan has one of the highest rates of malnutrition among children under five. About 2.1 million children under five are at risk of acute malnutrition. This includes 670 000 children who are severely wasted and 1.44 million who are…

DACULA, Ga., Nov. 13, 2025 (GLOBE NEWSWIRE) — What happens when poor dental hygiene is ignored for too long? According to Dr. Yadira Cardona-Rohena of Hamilton Mill Oral & Facial Surgery, the consequences can be severe and irreversible, often…

Unlock the Editor’s Digest for free
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
Scientists have developed a long-awaited anti-malaria medicine designed to counter rising drug resistance in Africa and…