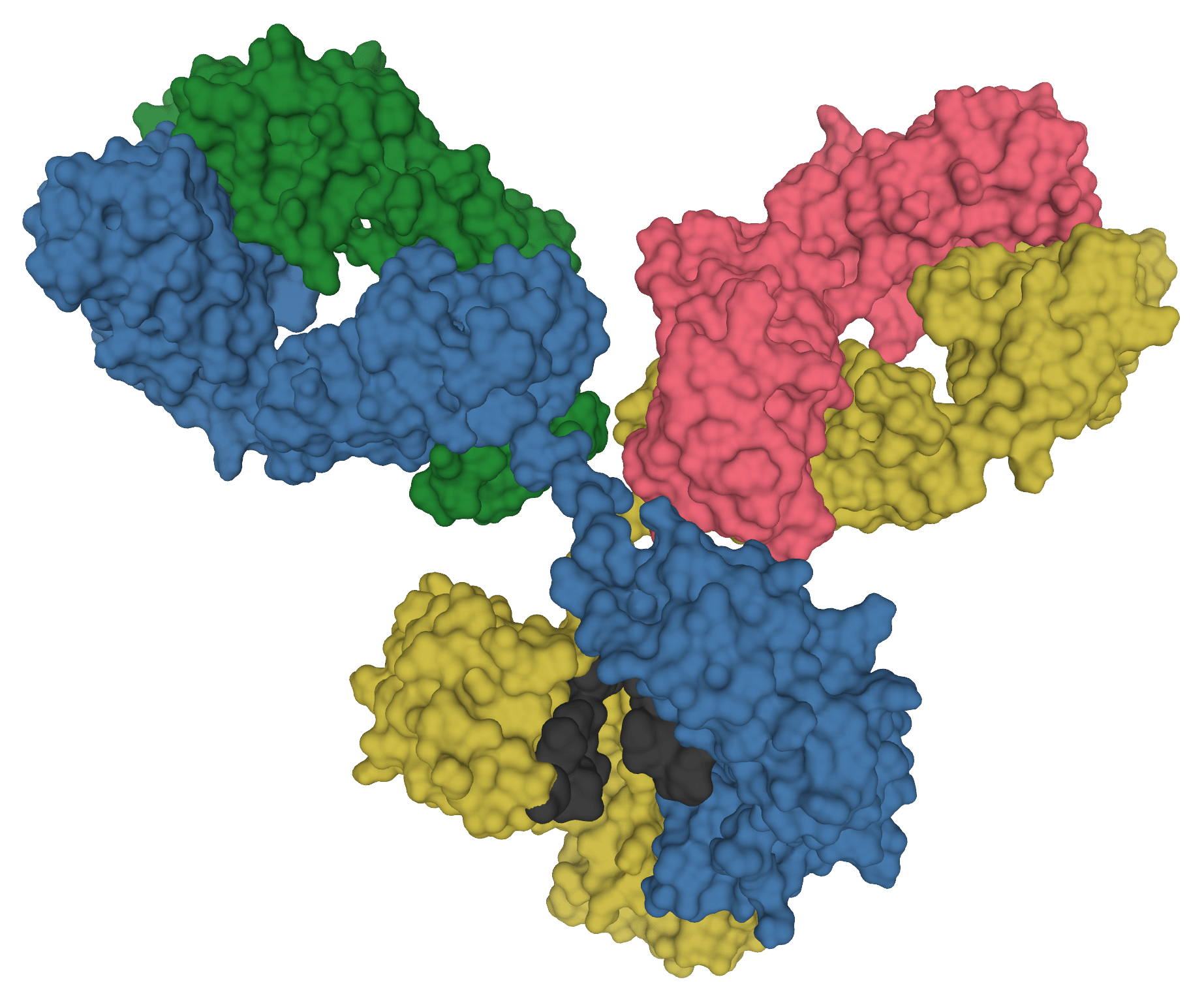
An antibody from a Tanzanian woman discovered during screening for anti-HIV antibodies shows a strong therapeutic potential in a preclinical study. Named 04_A06, it neutralised (blocked) 97.3% of over 300 HIV strains tested, and blocked 77% of…
MedPageToday The vaccine appeared to prompt responses to a range of H5N1 clades. An intranasal adjuvanted recombinant influenza vaccine appeared to safely prompt a robust response to a range of H5N1…