- DOH increases 2026 allocation for free measles, rubella jabs Philippine Information Agency
- Measles-rubella cases rise Daily Tribune
- ‘We don’t want to bury another child’: BARMM moves to vaccinate 577,000 children after deadly measles…
Category: 6. Health
-
DOH increases 2026 allocation for free measles, rubella jabs – Philippine Information Agency
-
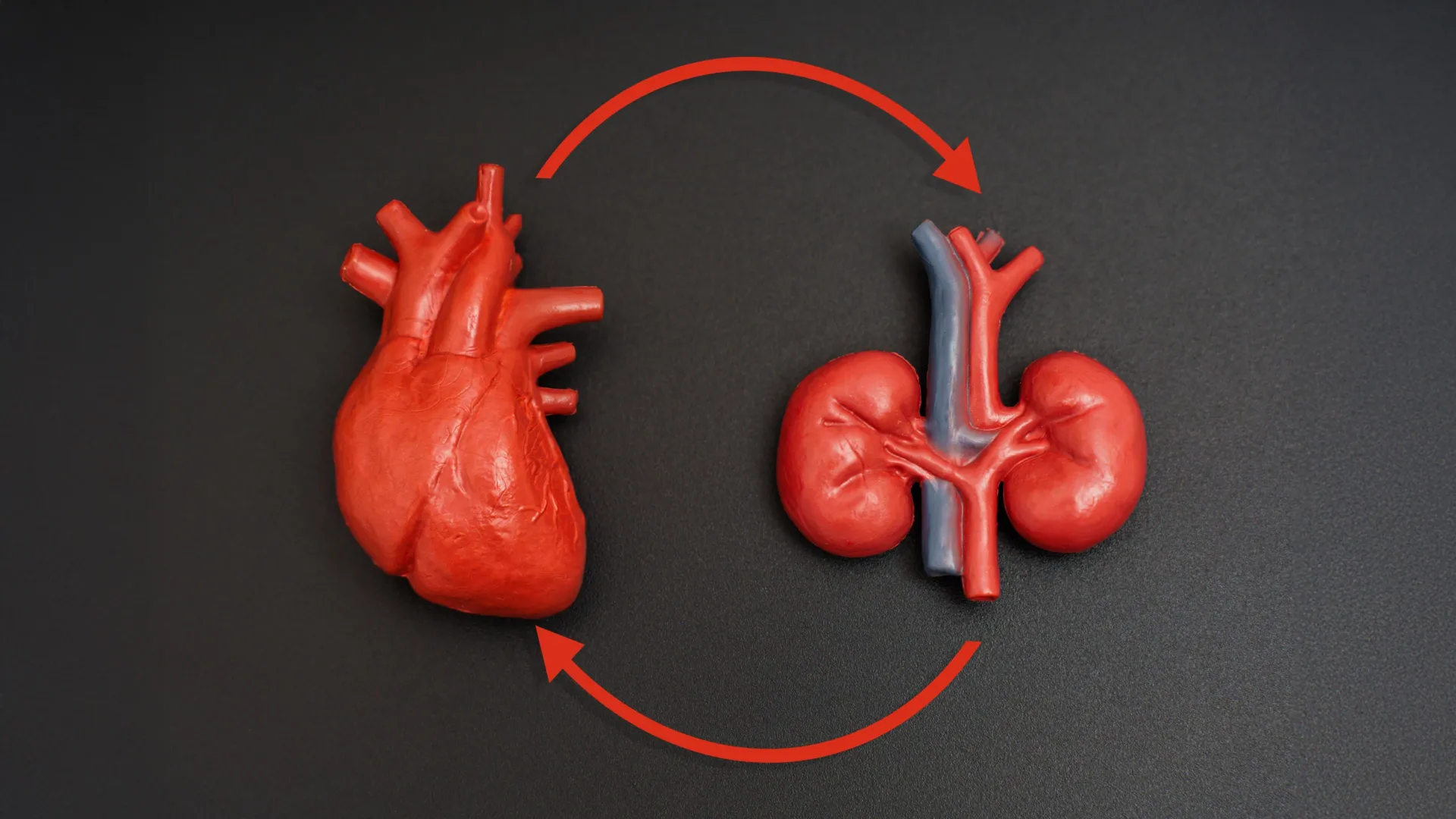
A little-known health syndrome may affect nearly everyone
A large majority of U.S. adults are unfamiliar with cardiovascular-kidney-metabolic (CKM) syndrome, even though it affects nearly 90% of adults nationwide. CKM syndrome is a recently defined condition that brings together several major health…
Continue Reading
-

Bird flu confirmed at Warwickshire’s Ryton Pools Country Park
Bird flu cases have been found at a Warwickshire beauty spot.
Ryton Pools Country Park, near Ryton-on-Dunsmore, highlighted the outbreak on Sunday via its social media channels.
A spokesperson for the site urged visitors to stick to footpaths,…
Continue Reading
-
Environmental metagenomics enhances detection of circulating viruses from live poultry markets in Cambodia
Peccia, J. et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020 38, 1164–1167 (2020).
Coker, R. J., Hunter, B. M., Rudge, J. W.,…
Continue Reading
-

Blood pressure medication linked with suicide risk? New study explains
Blood pressure medicines are among the most commonly prescribed drugs in the…
Continue Reading
-
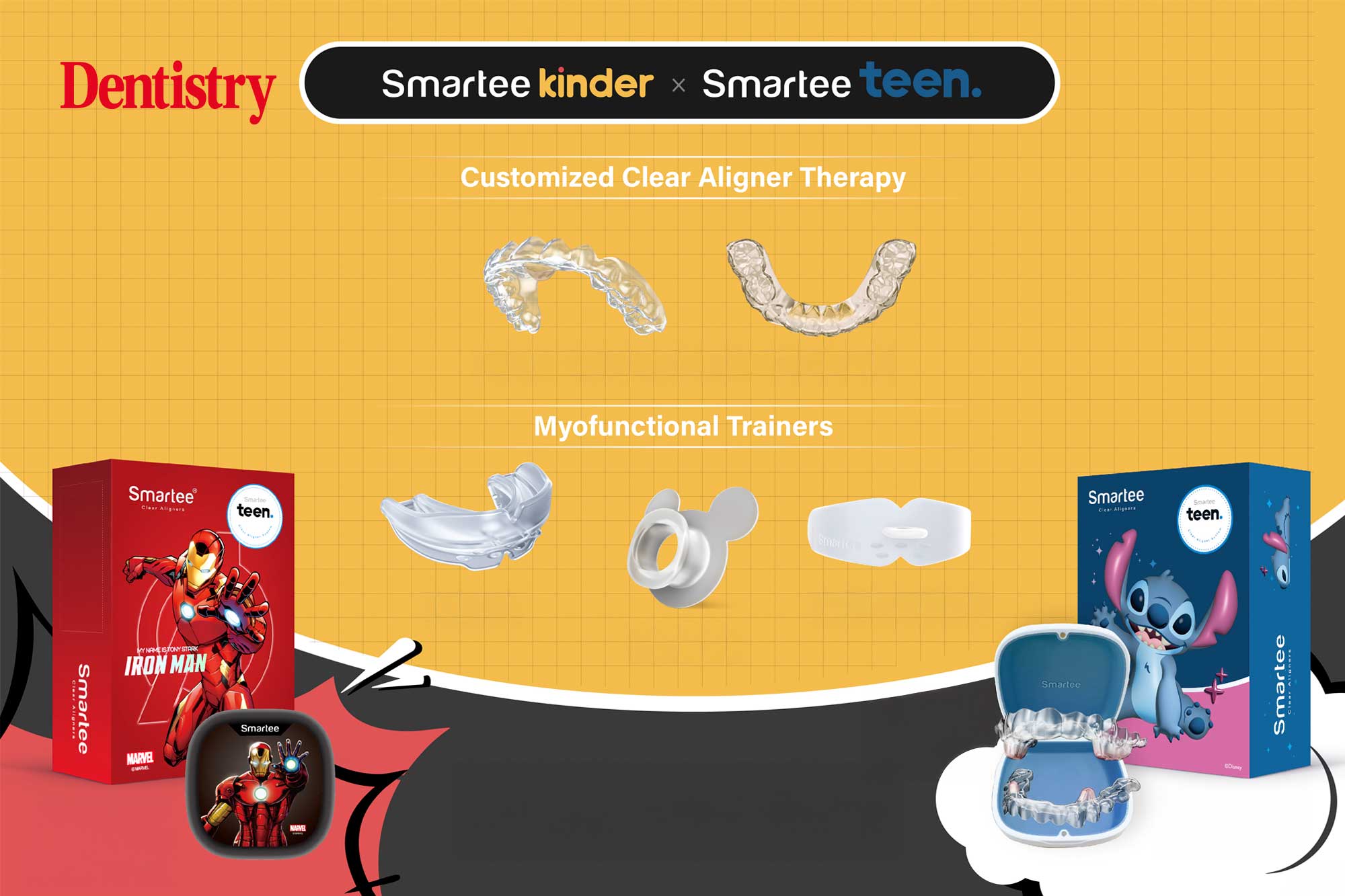
Smartee early orthodontic solutions: new designs
Smartee early orthodontic solutions are building integrated care from childhood onward, with two new packaging designs now launched.
Smartee officially introduced its new Disney-licensed Stitch…
Continue Reading
-

Life Events Prior to Alopecia Areata Onset — a Descriptive Cohort St
Introduction
Alopecia areata (AA) is an autoimmune hair loss disease often reported to be initiated by life events.1–4 AA is characterized by patches on the scalp that sometimes progresses to total loss of scalp hair, alopecia totalis (AT), or…
Continue Reading
-
Doctors say changes to U.S. vaccine recommendations are confusing parents and could harm kids | News, Sports, Jobs
FILE – Vaccines are prepared for students during a pop-up immunization clinic at a school in Louisville, Ky., on Thursday, Aug. 8, 2024. (AP Photo/Mary Conlon, File)