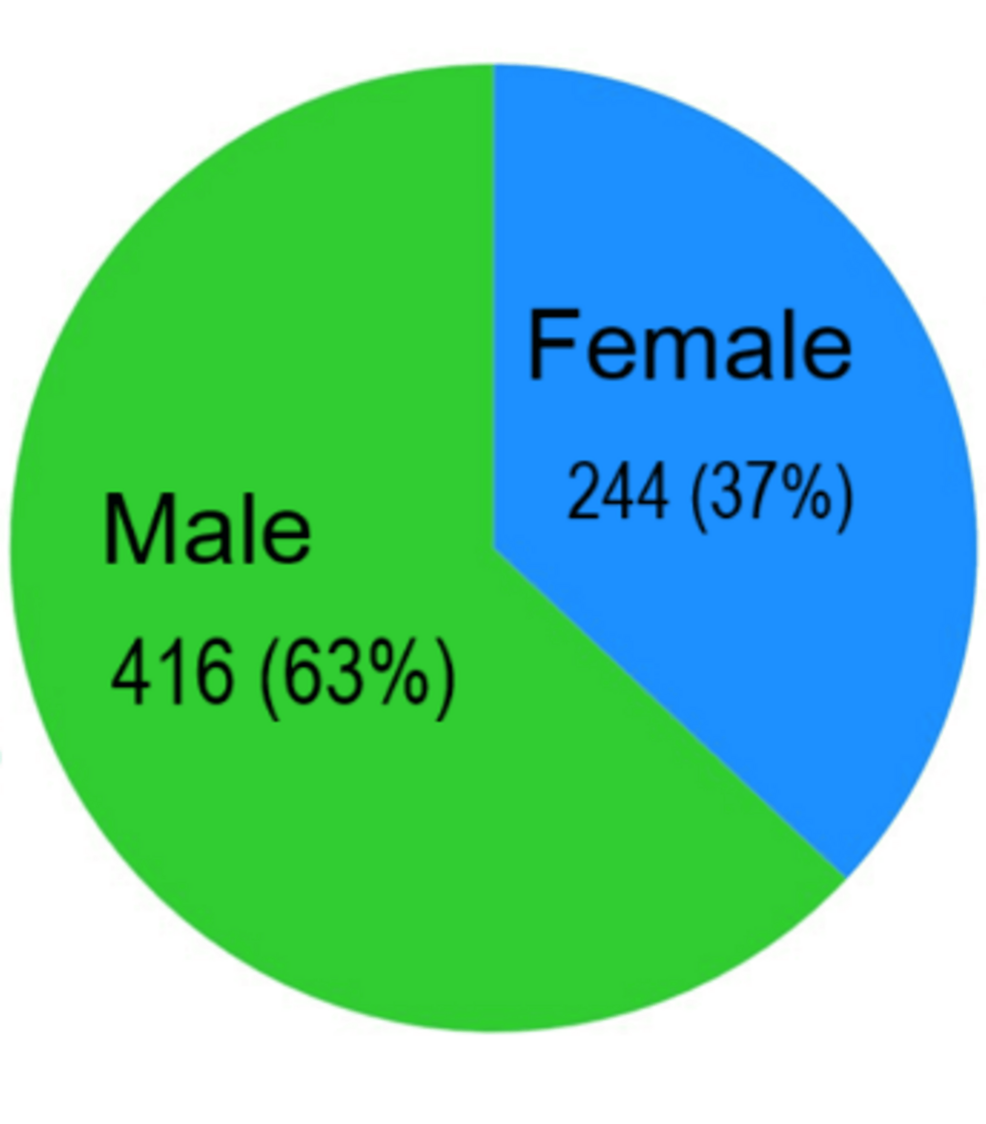
Category: 6. Health
-
Maternal Type 1 Diabetes Provides Epigenetic Protective Effect in Children – Genetic Engineering and Biotechnology News
- Maternal Type 1 Diabetes Provides Epigenetic Protective Effect in Children Genetic Engineering and Biotechnology News
- Blood methylome signatures in children exposed to maternal type 1 diabetes are linked to protection against islet autoimmunity
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-

Nanovaccine shows great promise for treating HPV-related cancers: Newsroom
Researchers tested the effectiveness of a nanovaccine in the lab to deliver antigens to immune cells and trigger the body’s protective response. (Photo credit: Getty Images)
DALLAS – Nov. 07, 2025 – A nanoparticle…
Continue Reading
-
Single-cell analysis reveals HPV-induced keratinocyte heterogeneity in cervical cancer
Cervical squamous cell carcinoma (CESC), the most prevalent subtype of cervical cancer, remains a major global health burden driven primarily by persistent high-risk HPV infection and genetic susceptibility.
A research team led by…
Continue Reading
-

Insurers on standby as flu cases surge across Asia
In Thailand, authorities documented more than 30,000 new flu infections within a single week. Japan, meanwhile, announced a nationwide influenza epidemic in early October after confirming over 6,000 cases in just…
Continue Reading
-
Steady glucose-monitor use helps blood sugar control
Adults with type 2 diabetes who consistently use continuous glucose-monitor devices experience significantly greater blood-sugar control than those who use such a device infrequently or not at all, new research shows.
The study, which…
Continue Reading
-
Mapping the brain’s body regulation and sensing system using 7 Tesla MRI
Jiahe Zhang, PhD, of the Department of Psychiatry at Mass General Brigham, is the lead author of the paper published in Nature Neuroscience, “Cortical and subcortical mapping of the human allostatic-interoceptive system using 7…
Continue Reading
-
Genomic mapping of resistance mutations in A. baumannii
The bacteria Acinetobacter baumannii (A. baumannii) is a haunting presence in many hospitals in the United States, where more than one in 100 patients are treated for A. baumannii infections. This species of bacteria is known for…
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading