- Child in Northern Virginia diagnosed with measles after international travel 13newsnow.com
- Confirmed case of Measles in Northern Virginia WWBT
- Virginia confirms second measles case of 2026; exposure sites listed WAVY.com
- Virginia’s second measles…
Category: 6. Health
-
Child in Northern Virginia diagnosed with measles after international travel – 13newsnow.com
-

Person with measles traveled through Maryland, Virginia last week, health officials say
The Maryland Department of Health said the person traveled on trains from Jan. 7-8.
A confirmed case of measles…
Continue Reading
-

39% of adults want to see ultra-processed foods banned – survey
Two thirds of UK adults believe the next generation will suffer poorer health due to ultra-processed foods (UPFs) and 39% would like to see them banned, a survey suggests.
Some 59% of adults believe UPFs are “impossible to avoid” when…
Continue Reading
-
39% of adults want to see ultra-processed foods banned – survey – ITVX
- 39% of adults want to see ultra-processed foods banned – survey ITVX
- How ultra-processed food is central to your diet The i Paper
- I was a UPF kid – this is how it has affected my health PressReader
- Two thirds fear ultra-processed foods will…
Continue Reading
-

39% of adults want to see ultra-processed foods banned – survey
UPFs often contain high levels of saturated fat, salt, sugar and additives, which experts say leaves less room in people’s diets for more nutritious foods (Alamy/PA) Two thirds of UK adults believe the next generation will suffer…
Continue Reading
-

Study links low lycopene intake to higher risk of severe gum disease in older adults

Photo: altitudevisual 123rf A new study from the US has found that insufficient dietary lycopene intake is associated with a significantly higher risk of severe periodontitis among US adults ages 65 to 79, with…
Continue Reading
-

Monika Pansari: Myths and Facts – Cervical Cancer Screening and the HPV Vaccine
Monika Pansari, Senior Surgical Oncologist at Manipal Hospitals (MHEPL), shared a post on LinkedIn:
“Cervical cancer is one of the most preventable cancers, yet many women still miss screening or delay vaccination – often…
Continue Reading
-
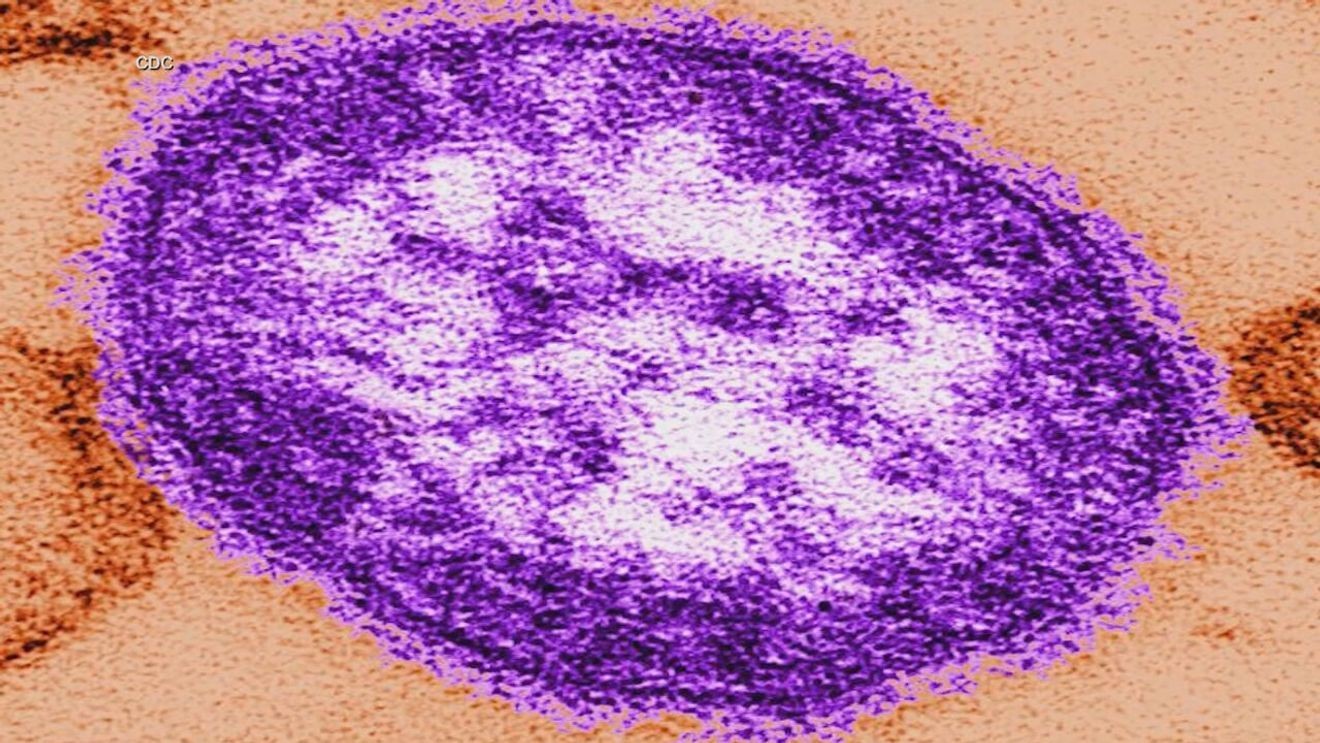
Possible measles exposure reported on Amtrak to DC, BWI shuttles in Maryland
WASHINGTON (7News) — The Maryland Department of Health (MDH) is warning the public of potential exposure after someone with a confirmed case of measles traveled through Washington, D.C., and Maryland while infectious in early January.
Health…
Continue Reading
-

Providence to restrict visitors under the age of 18 in hospitals and clinics in Spokane, Stevens counties | Spokane News
Providence to restrict visitors under the age of 18 in hospitals and clinics in Spokane, Stevens counties | Spokane News | khq.com
We recognize you are attempting to access…
Continue Reading
-

Increase in respiratory illnesses prompts visitor policy changes at Providence hospitals | News
Increase in respiratory illnesses prompts visitor policy changes at Providence hospitals | News | kxly.com
We recognize you are attempting to access this website from a…
Continue Reading