WASHINGTON (7News) — The Maryland Department of Health (MDH) is warning the public of potential measles exposure after someone with a confirmed case of measles traveled through Washington, D.C., and Maryland while infectious in early January….
Category: 6. Health
-
The diseases RFK Jr. says children don’t need to be routinely vaccinated for – Politico
- The diseases RFK Jr. says children don’t need to be routinely vaccinated for Politico
- CDC staff ‘blindsided’ as child vaccine schedule unilaterally overhauled The Washington Post
- CDC Urges ‘Shared Decision-Making’ on Some Childhood…
Continue Reading
-
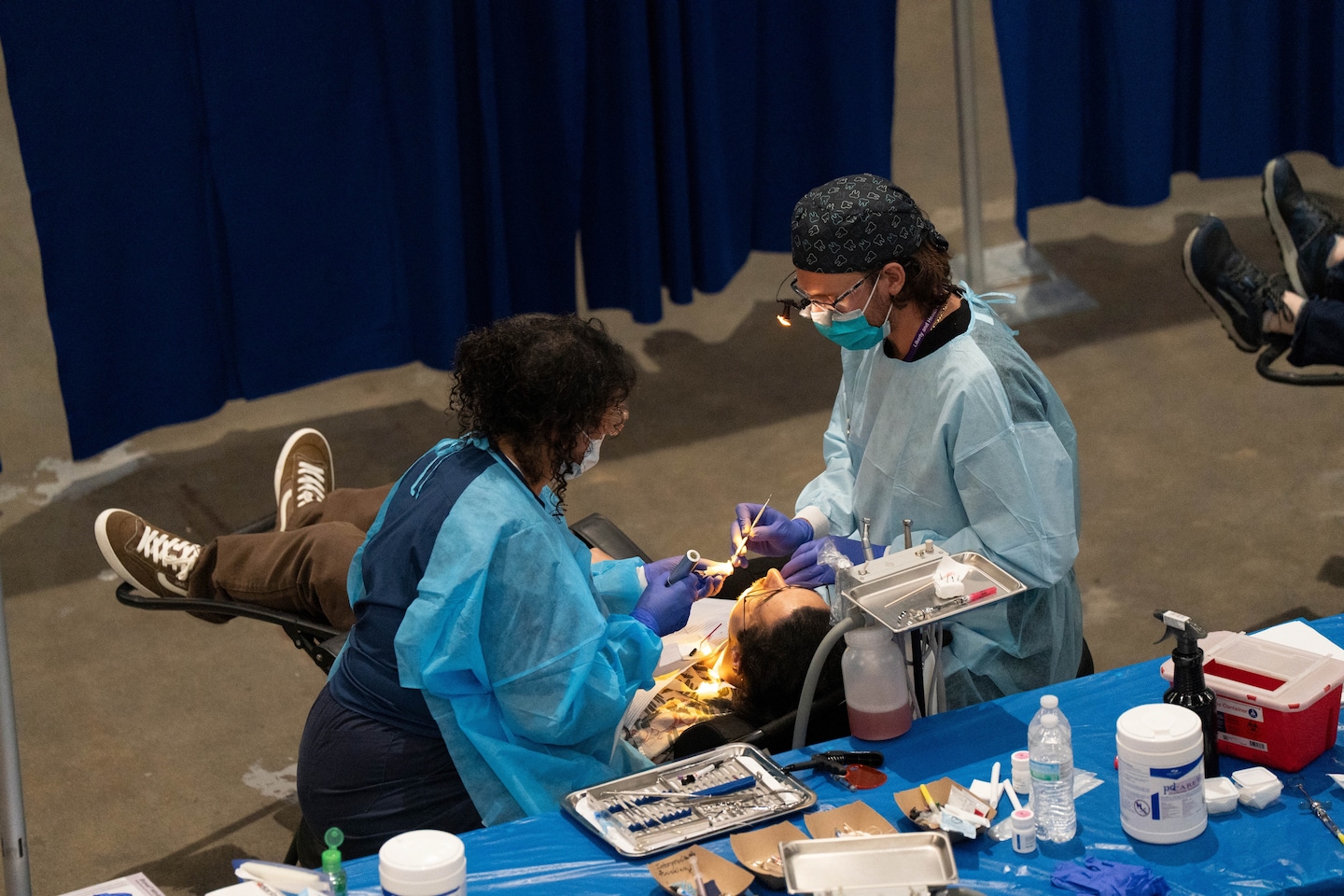
Dentists focus on getting young adults to stop skipping care
When Usman Ahmad talks to Gen Z patients about going to the dentist, he makes one case: “It’s about the future.”
If they don’t want a missing tooth, or a marred smile, or difficulty eating later in life, he tells them, keep coming back to…
Continue Reading
-

Raisa Mehzabeen: Nutrition and Oncology – A Critical Yet Underutilized Component of Cancer Care
“Cancer is not always a purely localized process; many cancers and many cancer treatments have systemic effects that disrupt metabolism, immunity, and functional capacity.
While modern oncology has made remarkable advances…
Continue Reading
-

Maryland Dept of Health issues notification of potential measles exposures associated with person who traveled through Maryland
Maryland Dept of Health issues notification of potential measles exposures associated with person who traveled through MarylandJanuary 11, 2026
Media Contact:
Amanda Hils, Assistant Director for Media Relations, [email protected]Baltimore,…
Continue Reading
-

25 Posts Not To Miss From ASCO GI 2026, part 2
The 2026 ASCO Gastrointestinal Cancers Symposium (ASCO GI 2026) is being held January 8–10, 2026, at Moscone West in San Francisco, California, with both in-person and online participation available for the global GI oncology…
Continue Reading
-
Study shows how fast kilos return after ending weight-loss drugs
When people stop taking the new generation of weight-loss drugs they pile back on the kilos four times faster than they would after ending diet and exercise regimes, according to new researchy.
But this was mostly because they lost so…
Continue Reading
-

Construction of a nomogram model for individualized prediction of risk
Introduction
Renal cell carcinoma (RCC) is a common malignancy, originating from the epithelium of the renal tubules and accounting for approximately 80% of malignant tumors of the kidney. It mainly affects middle-aged and elderly patients, and…
Continue Reading
-
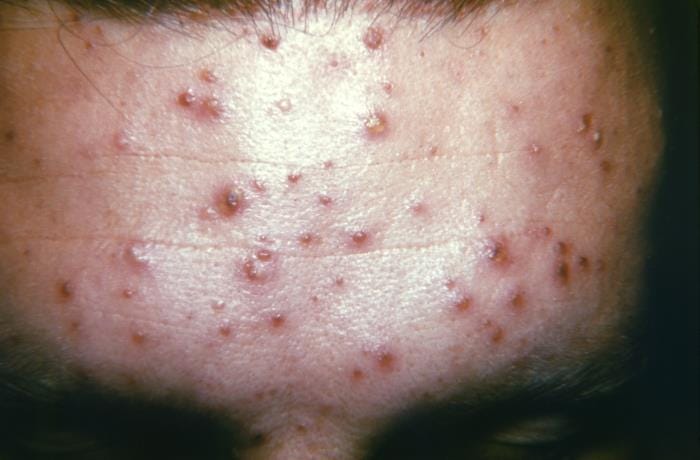
Chickenpox outbreak reported at Trinidad and Tobago prison
The Prison Officers’ Association (POA) posted the following on Friday:
There is an outbreak at the Maximum Security Prison of Chicken Pox. We advise our members to adhere to the strictest protocols to reduce the risk of transmission to your self…
Continue Reading
-
Have Flu Symptoms? Here Are 5 Things To Know, From An ER Doctor – Forbes
- Have Flu Symptoms? Here Are 5 Things To Know, From An ER Doctor Forbes
- Influenza Flu Symptoms May Be Changing As A New Variant Spreads: What To Expect Health and Me
- Patient First: Advice to protect yourself during flu season, and when it’s time…
Continue Reading