Most of us don’t think of sunlight as something that affects blood sugar. We think about food, exercise, and medicines. Light barely makes the list. But the body doesn’t see light as background scenery. It treats it like a signal. A strong…
Category: 6. Health
-

How a 1st year resident in St John’s helped solve a medical mystery, and didn’t stop there
Ian Gillies Sr. still can’t look at the pictures he took during his son’s two-month stint at the Health Sciences Centre in St. John’s.
Remembering what happened is enough for him, and for now — he’s trying looking ahead.
“We’ve been through hell…
Continue Reading
-

How intense workouts signal the bloodstream to suppress colorectal cancer genes- The Week
A new study published in the International Journal of Cancer has found that even brief bouts of intense physical activity can trigger molecular changes in the body that may help suppress the growth of colorectal cancer cells and accelerate DNA…
Continue Reading
-

a family’s shared calling to medicine
Dr. Dan Rorman never set out to create a family dynasty in medicine. Yet nearly three decades after opening a small community clinic in the Jerusalem-area town of Tzur Hadassah, he now sees all three of his daughters following him into the…Continue Reading
-

Leeds University moonsighting project unites science and religion
 University of Leeds/Mark Bickerdike
University of Leeds/Mark BickerdikeMuslim community leaders from across the UK will be taught to sight the moon The University of Leeds has been part of a pioneering project teaching the Muslim practise of moonsighting.
Moonsighters Academy is the…
Continue Reading
-

Impact of climate change on migraine frequency | barometric pressure triggers for migraine headaches | weather patterns and neurological pain symptoms | how extreme heat affects migraine sufferers | air quality and systemic inflammation migraine triggers | How climate change could be making your migraines worse
Migraines are not just bad headaches. They are a neurological condition marked by intense, throbbing pain often accompanied by nausea, and episodes of visual or sensory disturbances that signal a migraine attack.
Many people experience two to…
Continue Reading
-
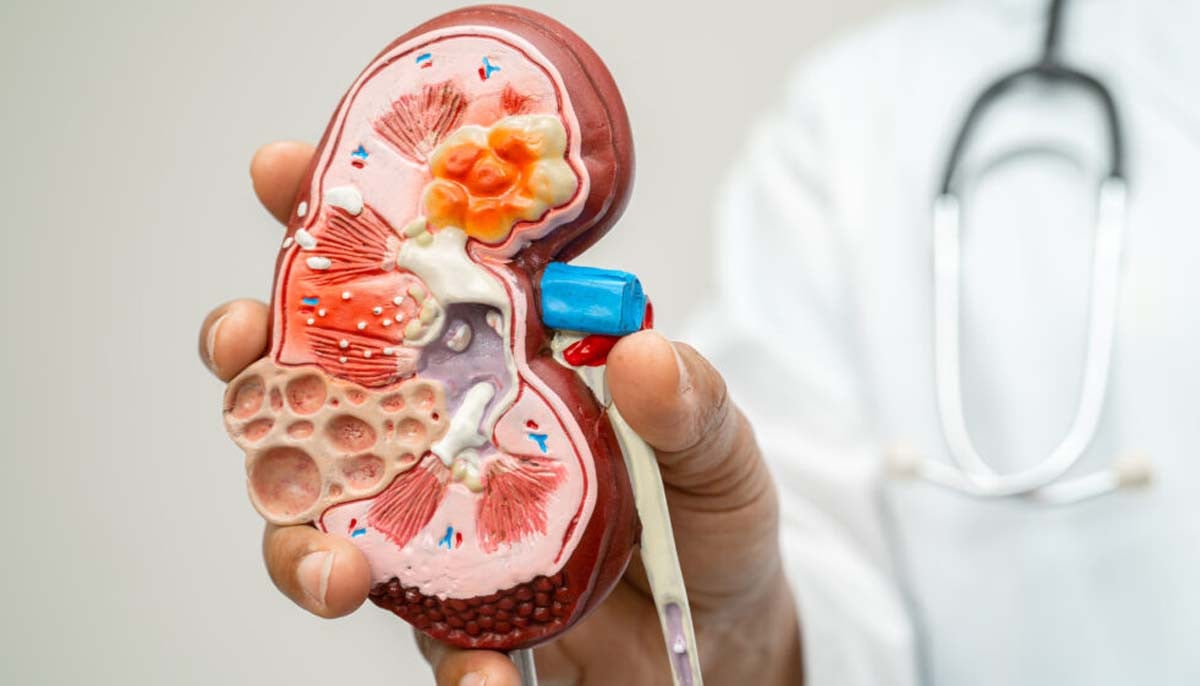
Kidney damage is now reversible: here’s where science stands
Acute kidney injury, or AKI, is a dangerous condition that affects many people in…
Continue Reading
-

Does intermittent fasting work for women same as men? Nutrition scientist reveals when food is delayed it impacts…
Intermittent fasting is often regarded as a valuable tool for weight loss and dieting. Many health influencers and experts often recommend intermittent fasting to individuals seeking to lose weight and burn excess fat. However, it is worth…
Continue Reading
-
A room full of flu patients and no one got sick
This year’s flu season has been especially harsh, driven in part by the rapid spread of a new variant known as subclade K. As cases rise, a newly released study offers surprising insight into how influenza spreads and how people may better…
Continue Reading