Tortora, S. C., Agurto, M. G. & Martello, L. A. The oral-gut-circulatory axis: from homeostasis to colon cancer. Front. Cell Infect. Microbiol. 13, 1289452 (2023).
Leiva-Sabadini, C., Saavedra,…
Tortora, S. C., Agurto, M. G. & Martello, L. A. The oral-gut-circulatory axis: from homeostasis to colon cancer. Front. Cell Infect. Microbiol. 13, 1289452 (2023).
Leiva-Sabadini, C., Saavedra,…
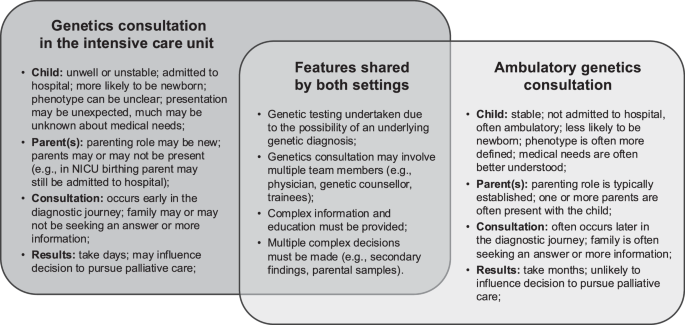
Kingsmore SF, Nofsinger R, Ellsworth K. Rapid genomic sequencing for genetic disease diagnosis and therapy in intensive care units: a review. NPJ Genom Med. 2024;9:17.
Google…

The review, commissioned by the trust in March 2025, found “clinical failures in the breast surgery service”.
The review also found “weaknesses in leadership, clinical governance, organisational culture and contract management over many years”.
In…

Bill EdgarLocal Democracy Reporting Service
 CDDFT
CDDFTAn NHS trust said it had taken “significant action” after systematic failings were found at…

The landscape of global health is changing rapidly driven by numerous…
COLOMBO, Jan. 10 (Xinhua) — Sri Lanka has recorded 2,170 dengue cases during the first nine days of 2026, health officials said on Saturday.
Prashila Samaraweera, community medical specialist of the National Dengue Control Unit, told…
An ITV doctor has claimed that there are a few things people who notice a problematic…

A 12-MONTH randomised trial suggests that two healthy dietary patterns, a polyunsaturated fatty acid–rich diet and a healthy Nordic diet, can significantly reduce liver fat in people with prediabetes or type 2 diabetes, even without…

NERATINIB markedly reduced vascular inflammation and atherosclerosis in mice by targeting ASK1, boosting effects with rosuvastatin.
Atherosclerosis begins with endothelial…

Listen to this article
Estimated 5 minutes
The audio version of this article is generated by AI-based technology. Mispronunciations can occur. We are working with our partners to continually review and improve the results.
Two people in Newfoundland…