Surprise, surprise: SpaceX shattered its single-year launch record again in 2025.
Elon Musk‘s company has now set a new mark six years in a row, and the numbers are getting pretty silly. The record has risen from 25 orbital liftoffs in 2020 to…

Surprise, surprise: SpaceX shattered its single-year launch record again in 2025.
Elon Musk‘s company has now set a new mark six years in a row, and the numbers are getting pretty silly. The record has risen from 25 orbital liftoffs in 2020 to…

Terms
While we only use edited and approved content for Azthena
answers, it may on occasions provide incorrect responses.
Please confirm any data provided with the related suppliers or
…

Researchers estimate that wildfires and prescribed burns release about 158 million tons of organic air pollutants each year.
A team in Beijing built the inventory to count heavier smoke gases from wildfires and prescribed burns that can form…

The attack, thought to have involved a juvenile great white which could have been around 4ft (1.2m) in length, took place in the early hours of 30 September.
It left Mr Murray with severe lacerations to his hand and foot, and meant he had to…

However, SKA leadership is at pains to stress that the scientific goals of the SKA will not be compromised, even though they may take substantially longer than initially envisioned. “The reason that we built the SKA is to be able to try to put…

Roughly 1,000 light-years away from Earth, a gigantic disk of gas and dust is swirling around a young star and giving rise to new planets. Not only is it the largest planet-forming disk astronomers have ever found, its behavior…
Here’s what you’ll learn when you read this story:
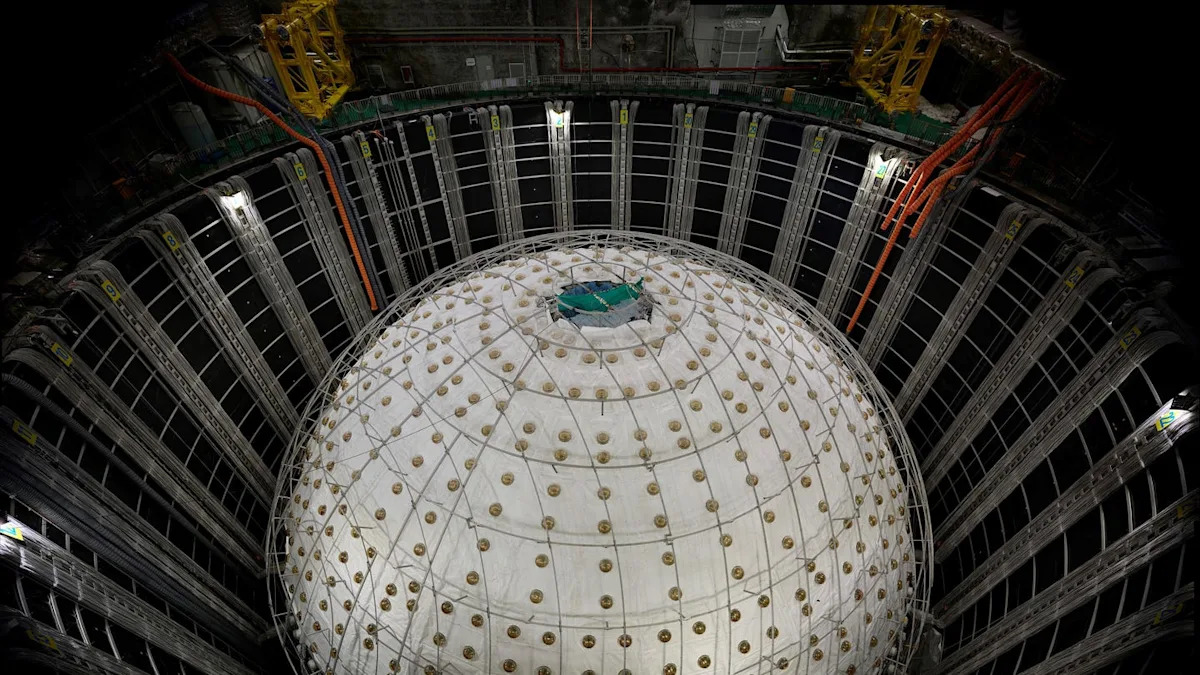
The Jiangmen Underground Neutrino Observatory (JUNO) is on the hunt to determine the mass ordering of neutrino flavors, and its first results show that it’s delivering beyond expectations.
Continue Reading


NASA’s Curiosity Mars rover used its black-and-white navigation cameras to…