Category: 7. Science
-

Chinese researchers develop AI model to process stellar data from different telescopes
Photo taken on June 19, 2015 shows the Large Sky Area Multi-Object Fibre Spectroscopy Telescope (LAMOST) at the Xinglong observation station of the National Astronomical Observatories under the Chinese Academy… -

NASA identifies astronaut with medical condition at space station – news.cgtn.com
- NASA identifies astronaut with medical condition at space station news.cgtn.com
- NASA’s SpaceX Crew-11 Astronaut Update NASA (.gov)
- Astronaut Mike Fincke reveals it was his medical issue that led to unprecedented early mission end CNN
- Agency…
Continue Reading
-
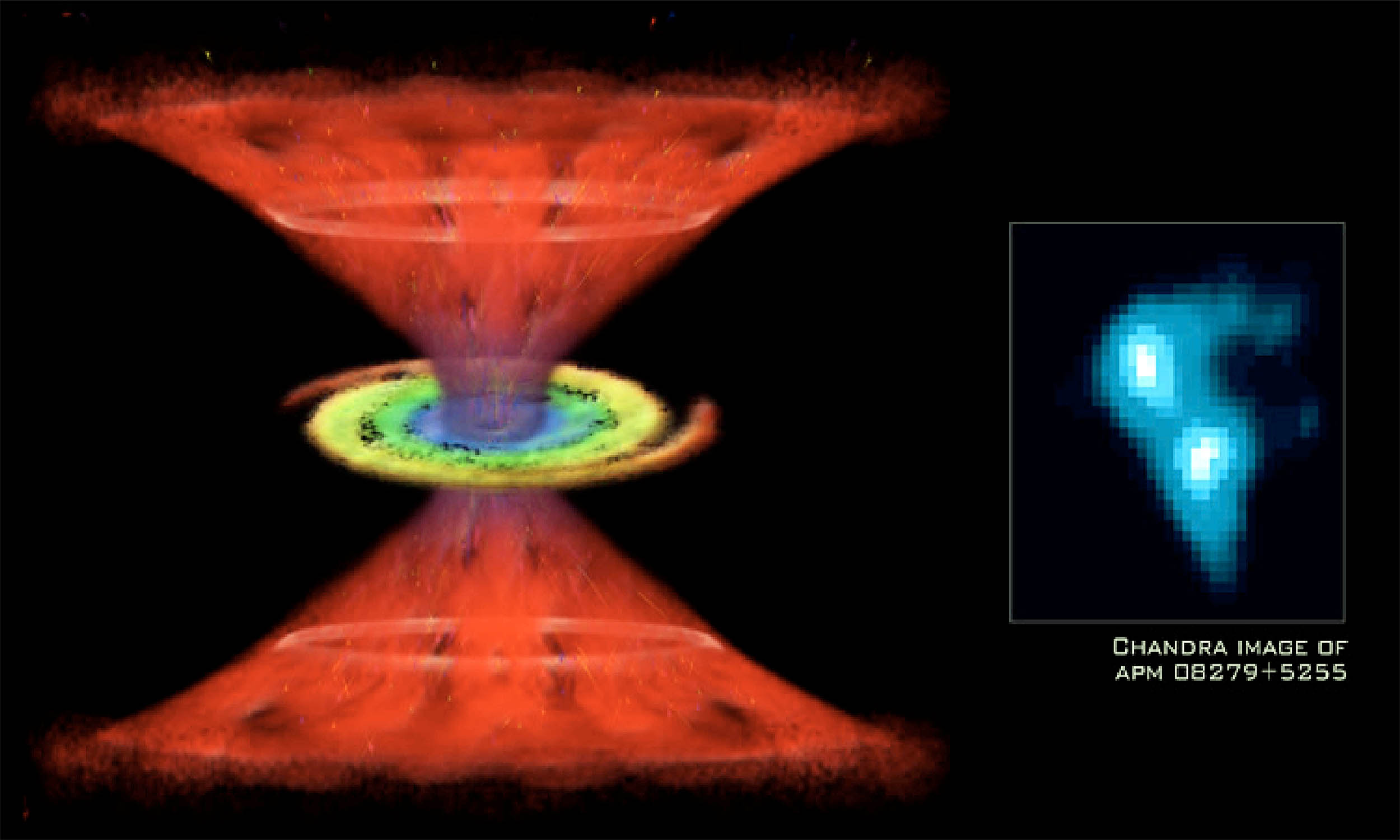
Black hole has enough water to fill “trillions of Earth-size oceans”
Astronomers enjoy it when the universe throws a curveball, and this object does exactly that. Working in two teams, they have found the largest, most distant stash of water ever seen in the cosmos. APM 08279+5255 is a quasar – an active galaxy…
Continue Reading
-

90-Million-Year-Old Patagonian Fossil Reveals Missing Chapter in Evolution of Alvarezsauroid Dinosaurs
A remarkably complete skeleton of the alvarezsauroid dinosaur species Alnashetri cerropoliciensis from Patagonia, Argentina, as well as two alvarezsauroid specimens from the northern hemisphere reveal how the once-mysterious lineage of…
Continue Reading
-

A cosmic explosion with the force of a billion Suns went unseen – until we caught its echo
Some of the universe’s most extreme explosions leave behind almost no trace. The original explosion is unseen, but our observations can capture the long-lived echo it leaves behind as the shock front ploughs into its surrounding…
Continue Reading
-
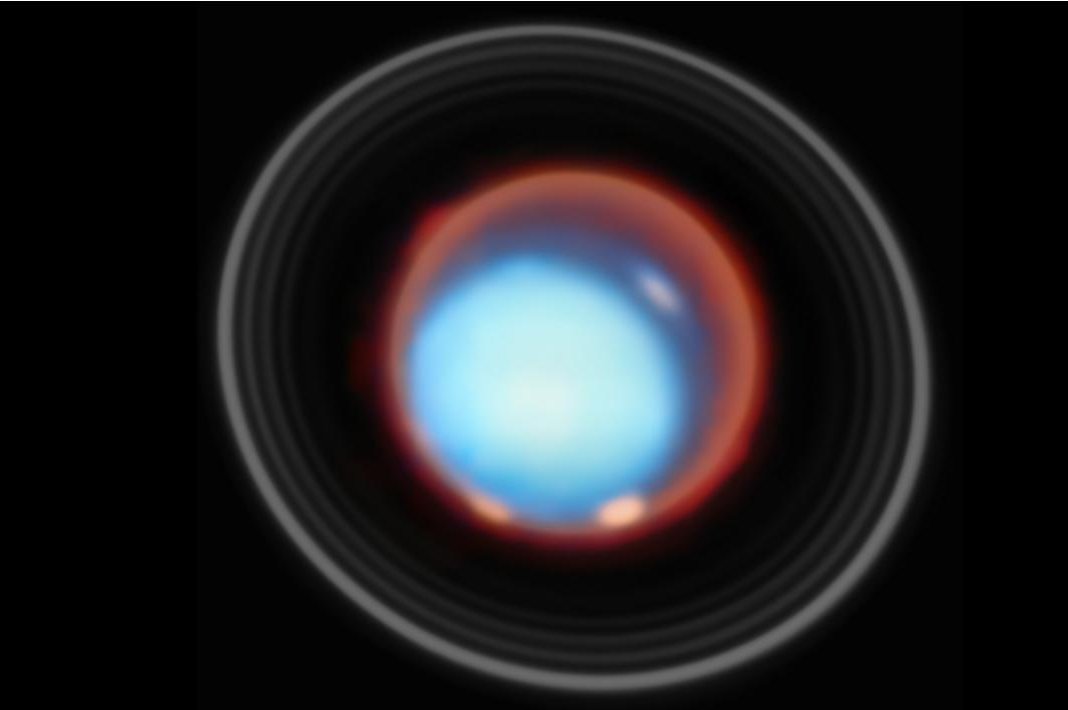
Webb telescope reveals Uranus’ upper atmosphere in unprecedented detail
NASA’s James Webb Space Telescope has captured the first vertical view of Uranus’s upper atmosphere, delivering an unprecedented look at the planet’s ionosphere and auroral structures.
The new observations, released by NASA on Tuesday, show…
Continue Reading
-

Squeaking at soft–rigid frictional interfaces
Branch, J. Why are basketball games so squeaky? Consider the spiny lobster. The New York Times (17 March 2017).
Rabinowicz, E. Stick and slip. Scientific American (1 May 1956).
Giannini, O., Akay, A. & Massi, F. Experimental analysis of brake…
Continue Reading
-

new survey of the sky will reveal the universe in unprecedented detail
When you look up at the night sky, it appears unchanging. But if you look deep enough you will find that the sky is in fact constantly shifting. Satellites, asteroids and interstellar objects pass by. Stars not only shine brightly, they can…
Continue Reading
-

NASA’s Aerospace Safety Advisory Panel Releases 2025 Annual Report
The Aerospace Safety Advisory Panel (ASAP), which advises NASA and Congress on safety, has released its 2025 annual report on NASA’s performance and challenges.
While the panel acknowledged NASA’s safety achievements, it warned that…
Continue Reading
-
Largest ALMA image ever shows cold gas in our galactic center – Astronomy Magazine
- Largest ALMA image ever shows cold gas in our galactic center Astronomy Magazine
- AP Trending SummaryBrief at 12:02 p.m. EST The News-Item
- World’s largest radio telescope array pierces heart of our Milky Way: ‘This is just the beginning’ Yahoo
Continue Reading