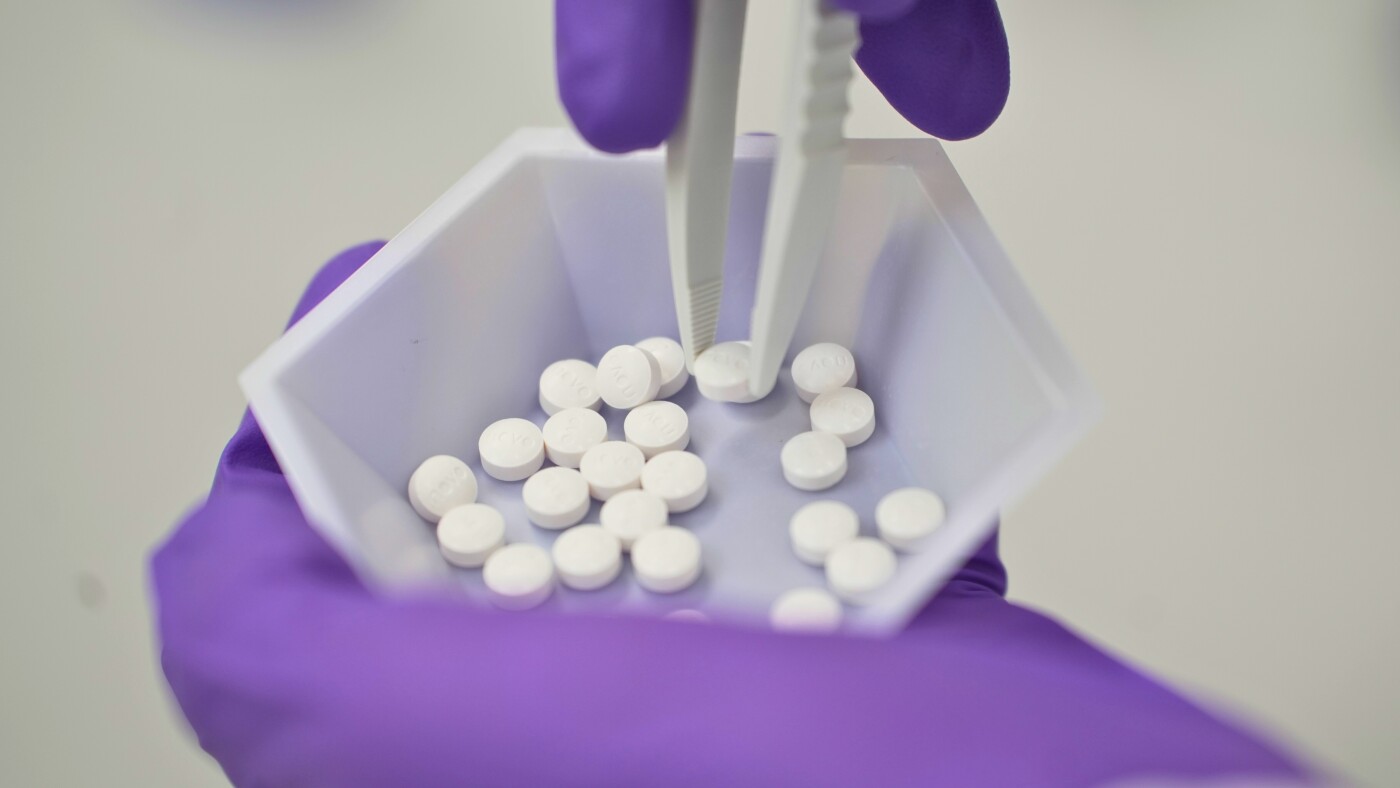
A pill form of Wegovy, the popular obesity drug previously available only by injection, is now being stocked by pharmacies.
Novo Nordisk
hide caption
toggle caption
Novo Nordisk
The Wegovy pill launched Monday, and the starting dose is now available at pharmacies around the country with higher doses arriving by the end of the week.
The Food and Drug Administration approved the pill for obesity on Dec. 22. It’s also approved to reduce the risk of heart attack and stroke in patients who are obese or overweight.
The pill follows the blockbuster success of Novo Nordisk’s Wegovy injection, which has been on the market since 2021 and became so popular that it was in short supply until February 2025.
The pill version of the drug helped patients lose about as much weight as the injection. In a study published in the New England Journal of Medicine, a 25 milligram Wegovy pill led to a 13.6% reduction in weight on average over 64 weeks. Patients taking a placebo in the study lost 2.2% of their weight. For patients who stayed on the treatment, reduced their calorie intake and exercised, Novo Nordisk estimates they would have a 16.6% reduction in their weight.
However, patients need to take the Wegovy pill on an empty stomach and wait a half hour before eating anything else for the medicine to be properly absorbed. The most common side effects with the Wegovy pill are similar to the injection and include nausea, diarrhea and vomiting.
When Novo Nordisk announced its drug-pricing deal with the Trump administration in November, it promised to make the obesity pill available for $149 a month to patients not using their health insurance. However, that’s only the starting dose for the direct-to-consumer price. The higher doses will be available for $299 a month.
The price that affects insurance coverage, called a list price, is the same as the Wegovy injection: $1,349 a month.
Insurance coverage for obesity drugs became more restrictive in 2025, according to an analysis from GoodRx, a website that helps patients find discounts on prescription drugs. But Novo Nordisk says patients with insurance coverage can get the Wegovy pill for as low as $25 a month.
Although the Wegovy pill is the first of its kind to win FDA approval, Novo Nordisk’s Type 2 diabetes pill, Rybelsus, is already on the market. It contains the same active ingredient, semaglutide, but the doses are different.
Eli Lilly, which makes the Zepbound injection, applied to the FDA in late 2025 for approval of its competing obesity pill. The agency gave the company a voucher for a priority review and a decision could come early this year.