Atrial Fibrillation Ablation
Catheter ablation has become an effective treatment strategy for patients with AF. In 2009, the ThermoCool AF (NAVISTAR® THERMOCOOL® Catheter for the Radiofrequency Ablation of Symptomatic Paroxysmal Atrial…

Atrial Fibrillation Ablation
Catheter ablation has become an effective treatment strategy for patients with AF. In 2009, the ThermoCool AF (NAVISTAR® THERMOCOOL® Catheter for the Radiofrequency Ablation of Symptomatic Paroxysmal Atrial…

A study co-led by researchers at the University of Hawaiʻi at Mānoa has found that exposure to per- and polyfluoroalkyl substances (PFAS)—commonly known as “forever chemicals”—may significantly increase the…
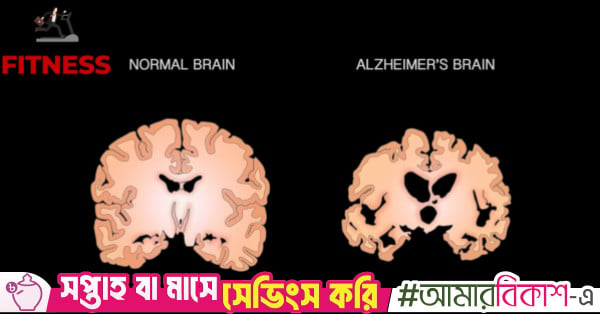
Alzheimer’s disease and dementia don’t suddenly appear in old age – they are often the result of decades of small, everyday habits quietly adding up over time.
The choices you make today around food, sleep, movement, and inflammation can…

Chronic Wasting Disease (CWD) has been reported in a hunter-harvested white-tailed buck in Concordia Parish, the Louisiana Department of Wildlife and Fisheries (LDWF) said. The buck was harvested on Richard K. Yancey Wildlife Management…
The Ministry of Health announced that no new cases of Marburg Virus Disease (MVD) have been detected in Ethiopia over the past 21 days, signaling progress in containing the outbreak, though authorities warned that the outbreak will only be…

The SEER-MHOS 2026 Update: Your Guide to the New Data Linkage is a specialized technical webinar hosted by the National Cancer Institute (NCI) Division of Cancer Control and Population Sciences. Scheduled for January 15,…
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Hopstaken, J. S….

Findings published in a special issue of ADLM’s Clinical Chemistry journal focused on perinatal diagnostics
WASHINGTON , Jan. 6, 2026 /PRNewswire/ — A new study indicates that…

A review and meta-analysis of more than 200 studies shows a “persistently high” prevalence of bacterial sexually transmitted infections (STIs) in prisons, particularly among incarcerated adolescents and women, researchers…