Study design and participants
This was a prospective, multi-center, exploratory, cross-sectional study. The study protocol was approved by the Institutional Review Boards of Seoul National University Hospital (IRB No. 2310-095-1476) and Dongguk…
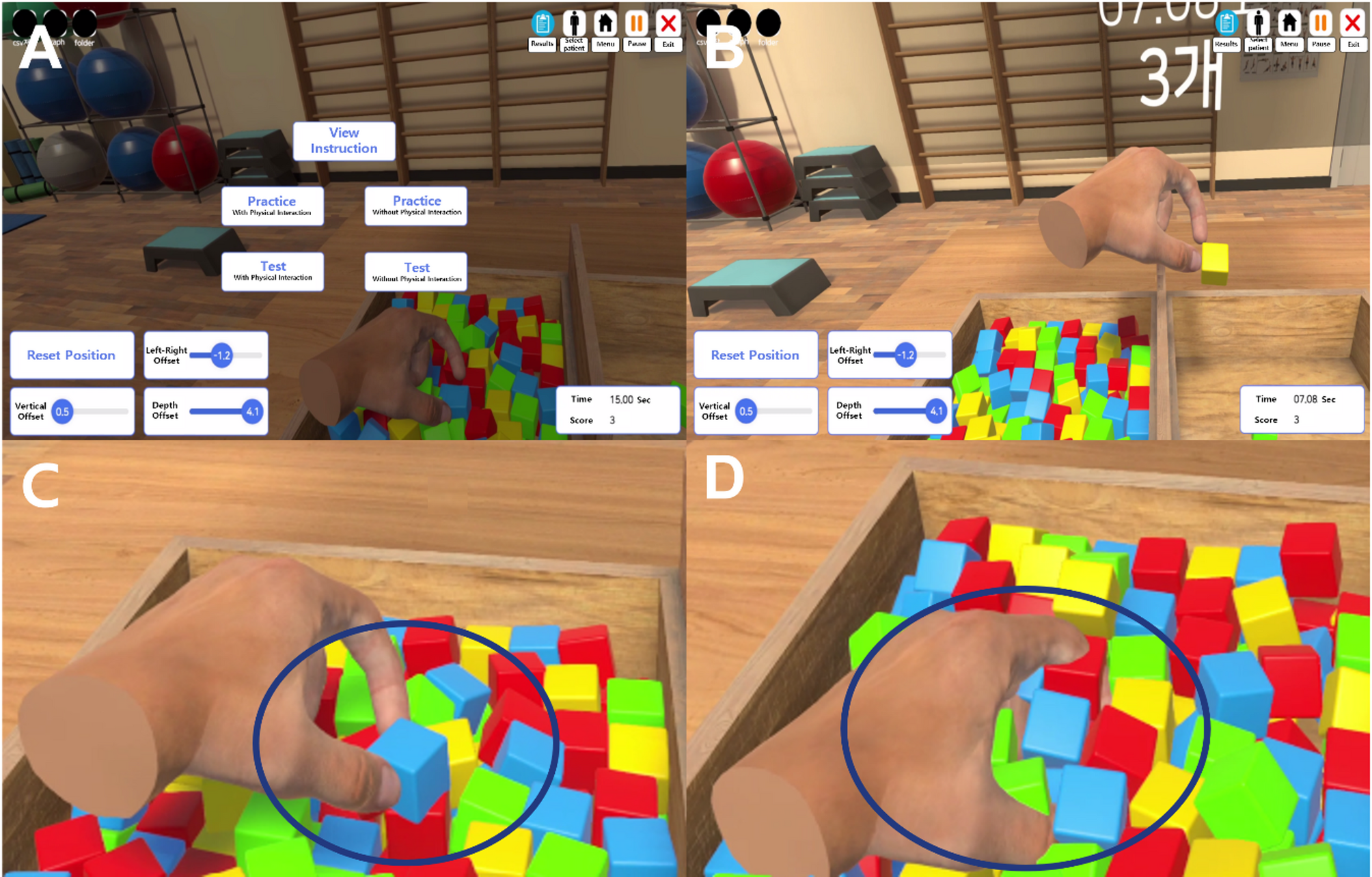
This was a prospective, multi-center, exploratory, cross-sectional study. The study protocol was approved by the Institutional Review Boards of Seoul National University Hospital (IRB No. 2310-095-1476) and Dongguk…
A new 20-year study of nearly 11,000 adults in Bangladesh found that lowering arsenic levels in drinking water was associated with up to a 50 percent lower risk of death from heart disease, cancer and other chronic illnesses,…
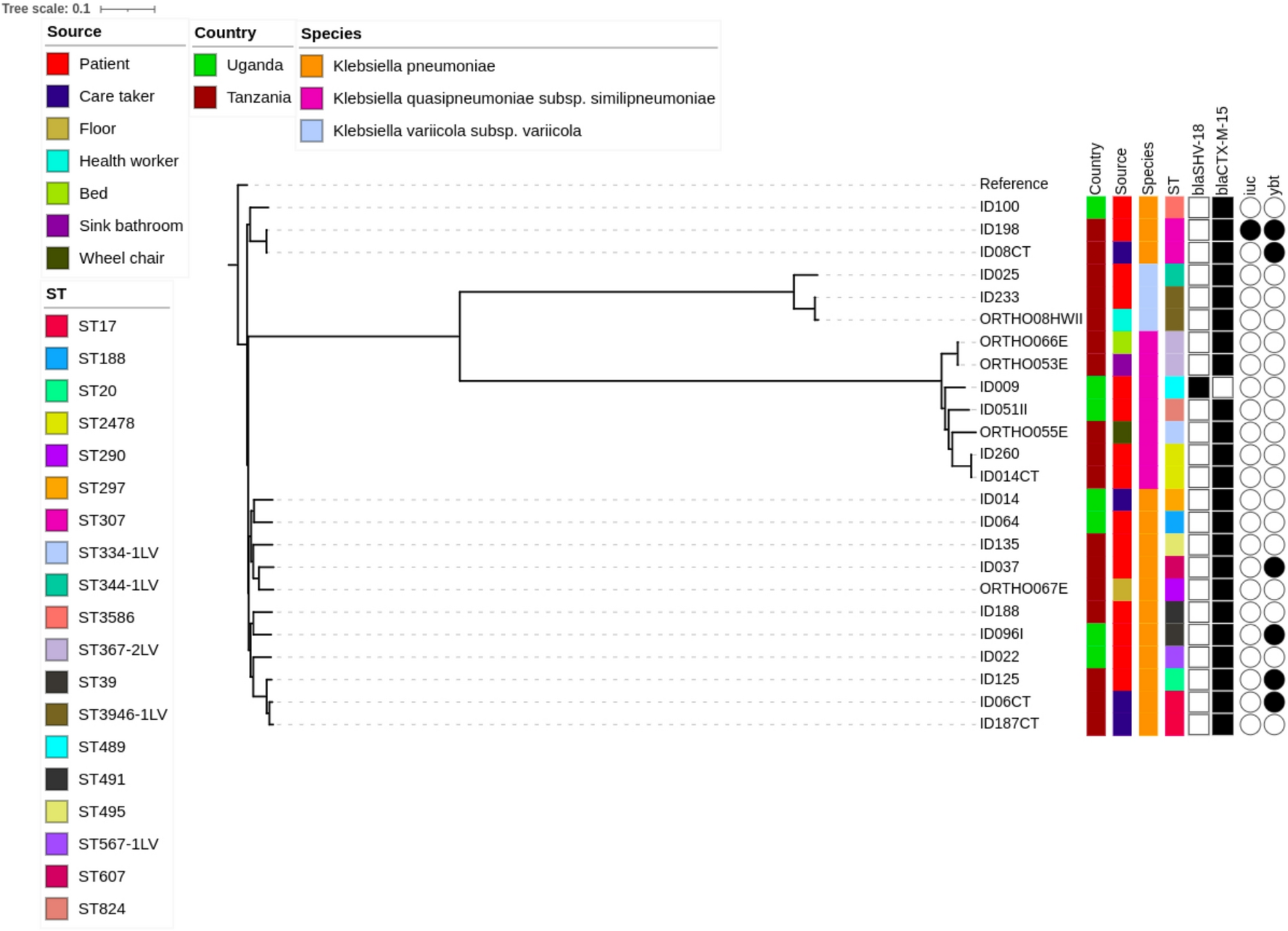
A total of 24 ESBL-KpSC isolates were analyzed. Of these, 15 (62.5%) were obtained from orthopedic patients with open fractures, four (16.7%) from…
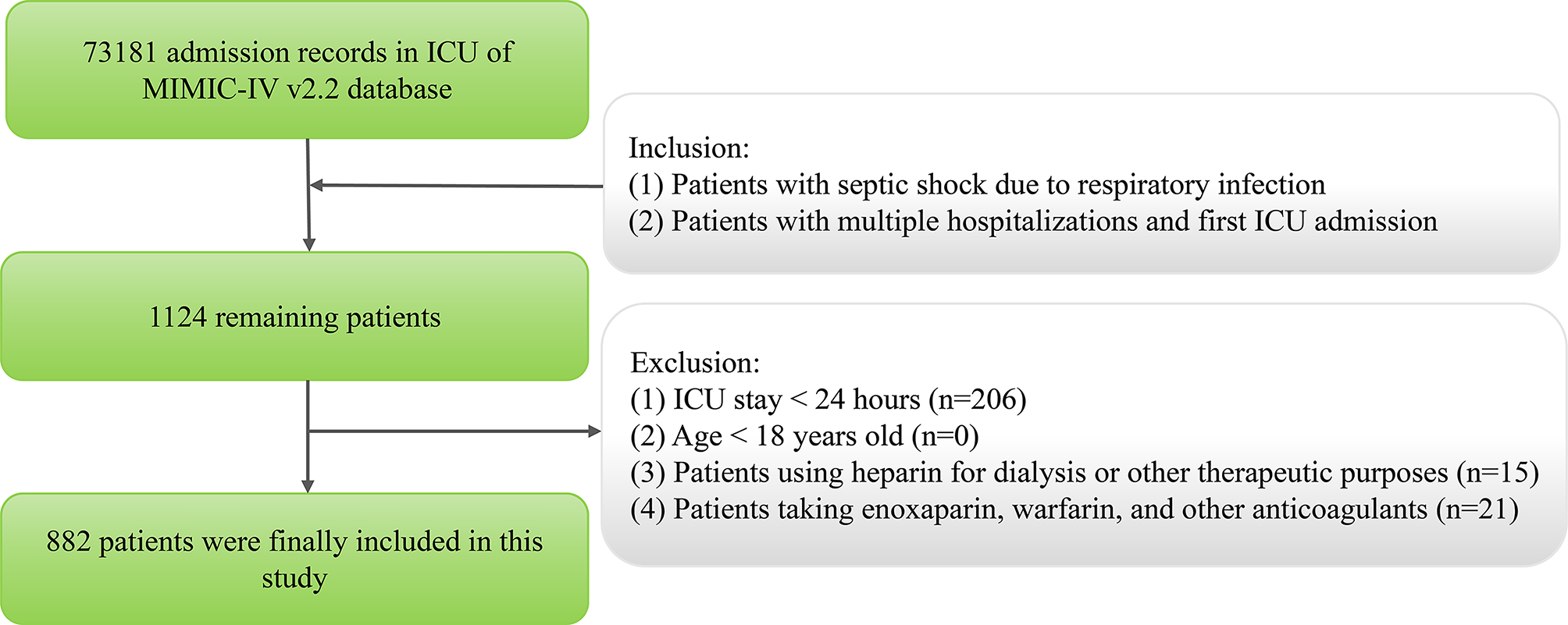
Mahapatra S, Heffner AC. Septic Shock. In StatPearls. 2025.
Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a…
Roughly a quarter of all cancerous tumors are caused by mutations in the KRAS gene, which fosters cell growth. For more than three decades, scientists believed these mutations were impervious to treatment. Today, National Institutes of Health…

Timely and evidence-informed action is critical for responding to public health threats. The COVID-19 pandemic highlighted this need, resulting in a growing demand among public health professionals to work in a more data-driven manner…

Modification of perioperative anti-rheumatic medication as recommended in guidelines for total hip and knee arthroplasty was an effective solution to reduce early postoperative wound complications in patients undergoing total ankle arthroplasty…

MIAMI, Nov. 17, 2025 /PRNewswire/ — Resolve Therapeutics, a mid-stage clinical biopharmaceutical company pioneering non-immunosuppressive drugs for inflammatory diseases today announced it will highlight its platform…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!