Cardiovascular disease is one of the most prevalent causes of mortality, globally.
getty
A new study by the University of Bonn found that moderate oatmeal consumption, even for just two days, was associated with significant decreases in patient…

Cardiovascular disease is one of the most prevalent causes of mortality, globally.
getty
A new study by the University of Bonn found that moderate oatmeal consumption, even for just two days, was associated with significant decreases in patient…
You…

The benefits of a vegan diet are well documented. From promoting a healthy weight and reducing our risk of heart disease and type 2 diabetes, to improving gut health and lowering blood pressure, countless studies have found good reason to consume…
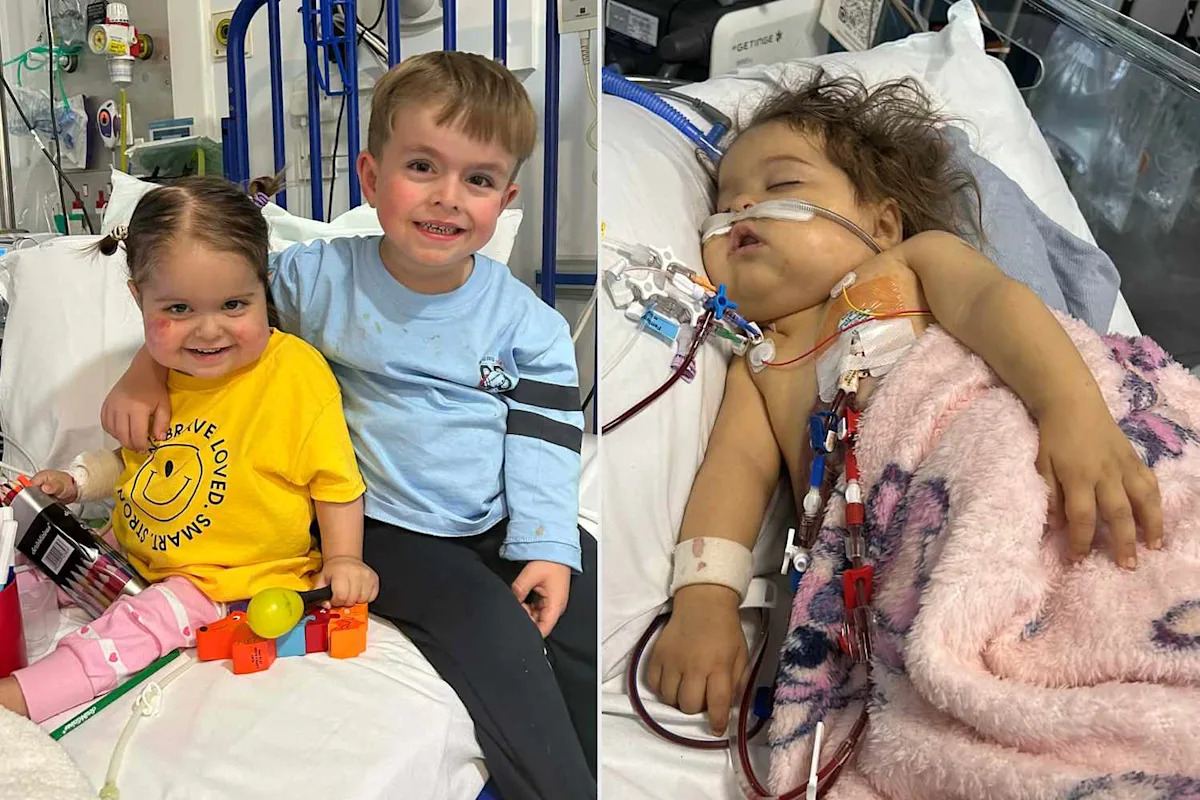
Etta Cartmill, a 3-year-old girl in Northern Ireland, is preparing for her second major organ transplant
The child was born with an extremely rare genetic condition known as TTC21B
The girl’s older brother, Olly, was also born with…
Aging rewires the jobs your cells do, and that slow transformation can result in scarring, weakness, and organ failure.
Scientists now see a way to push some cells back toward healthier roles, raising hopes for treating age-linked diseases.

| DAYS | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| MEALS | Breakfast: Avocado Toast w/ Jammy Eggs ——– Lunch: Greek Chopped Salad w/ Chicken ——– Dinner: Broiled Salmon & Cabbage Salad |
Breakfast: Tofu & Vegetable Scramble ——– Lunch: Chicken & Rice Soup +… |
WARSAW, Jan. 24 (Xinhua) — Poland’s largest population genomics initiative, “Genomics for Poland,” was launched on Saturday in Poznan, according to the Polish Press Agency.
Led by the Institute of Bioorganic Chemistry of the…
WARSAW, Jan. 24 (Xinhua) — Poland’s largest population genomics initiative, “Genomics for Poland,” was launched on Saturday in Poznan, according to the Polish Press Agency.
Led by the Institute of Bioorganic Chemistry of the Polish Academy…
A recent study led by the CDC reveals that routine vaccinations do not raise epilepsy risk in children, alleviating concerns over immunisation safety.
A comprehensive study led by the Centres for Disease Control and Prevention (CDC) in the…