Background and aims
Bacterial infections (BIs) are common and severe complications in patients with liver cirrhosis, but global data are limited. Here, we aimed to evaluate the global prevalence, temporal changes, and associated…
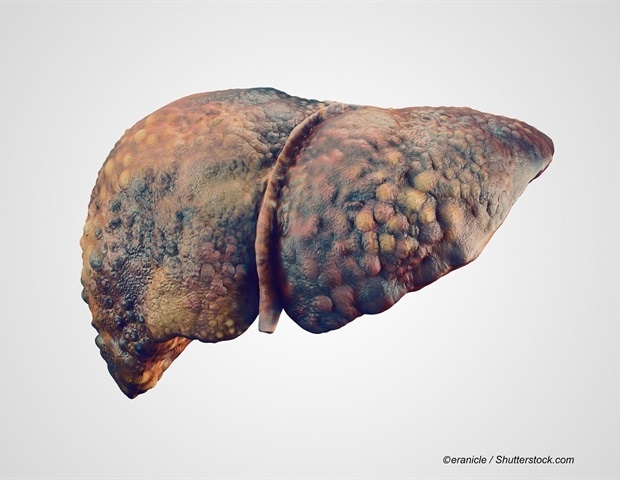
Bacterial infections (BIs) are common and severe complications in patients with liver cirrhosis, but global data are limited. Here, we aimed to evaluate the global prevalence, temporal changes, and associated…
According to the Ho Chi Minh City Center for Disease Control (HCDC), 5,228 dengue fever cases have been reported, including 1,430 dengue fever cases in the city over the past week.
Binh Hung Hoa, Tay Nam and An Nhon Tay are the wards and communes…

A November South Australian study published in Economic Analysis and Policy found that diet and exercise habits seldom change after retirement, citing a need for healthy lifestyle habits during an individual’s working life to…

A long-term study published in Science Advances has found that while a ketogenic diet limited weight gain in mice, prolonged adherence triggered metabolic disruptions, including impaired insulin secretion, fatty liver disease and elevated blood…

The use of antiviral treatments in children hospitalized for influenza fell sharply during the COVID-19 pandemic, according to a study published last week in Pediatrics.
While 48% to 57% of hospitalized children…
Researchers don’t have a definitive answer on what’s behind young people developing colorectal cancer, but the evidence right now points to lifestyle and environmental factors.
Another factor, he told Healthline, is that the colon is uniquely…

Terms
While we only use edited and approved content for Azthena
answers, it may on occasions provide incorrect responses.
Please confirm any data provided with the related suppliers or
…