Add your e-mail address to receive free newsletters from SCIRP.

The transaction was structured as a share acquisition of minority shareholding in the vessel-owning companies of eight 174,000 cbm LNG carriers, each valued at approximately US$230m. All of the eight LNGCs in this project are financed respectively with three prominent PRC lessors and on long term time charters to affiliates of an integrated energy company in the Middle East. Completion of the transaction is expected in Q1 2026.
Founded in 2012, CMC is a member of the China Merchants Group and a leading Chinese alternative investment headquartered in Shenzhen and Hong Kong.
The cross-border WFW maritime team advising CMC was led by Hong Kong Office Head Guan Jian and London Partner Mark Tooke, supported by Registered Foreign Lawyer Catherine Tse, Associate Annie Tsang and Senior Legal Manager Jasmine Kwok and Legal Manager Sean Feng in Hong Kong Office, and Associates Idil Yusuf, Jonathan Ford and Megha Vijh in London Office; London Partner Solange Leandro, supported by Senior Associate Edita Katuscakova, advised on the merger control aspect of the transaction; London Partner Richard Stephens advised on tax matters; London Partner Joe McGladdery supported by Associate Amelia Reffold provided specialist’s advice on LNGC time charters; New York Partner Daniel Pilarski provided specialist’s advice on USTR port fees.
Guan Jian commented: “We are delighted to have supported CMC on these landmark shipping joint venture transactions which reaffirms both the strategic importance of LNG in the global energy transition and the strength of our cross-border capabilities in servicing different stakeholders in the LNG sector. This deal highlights WFW’s ability to deliver seamless advice across jurisdictions and practice areas, ensuring our clients achieve their commercial objectives in complex, high-value transactions”.
WFW has advised clients on a broad spectrum of financing structures involving LNG carriers. These joint venture transactions not only underscore the firm’s capability to deliver sophisticated legal services across multiple sectors but also reaffirm our specialised expertise in the LNG market and the confidence our clients continue to place in us in this field. Our active role in this sector is further highlighted by our various mandates in recent years advising different stakeholders involved in the same Middle East charterer’s LNG fleet expansion initiatives.

Traders work on the floor at the New York Stock Exchange (NYSE) in New York City, U.S., Jan. 28, 2026.
Brendan Mcdermid | Reuters
Stock futures fell on Sunday night as Wall Street begins a new month of trading, with traders keeping an eye on bitcoin after a weekend sell-off.
Dow Jones Industrial Average futures lost 143 points, or 0.3%. S&P 500 futures dipped 0.6%, while Nasdaq-100 futures shed nearly 1%.
Bitcoin dropped below $80,000 for the first time since April, a sign investors were taking more risk off the table following Friday’s sharp declines in gold and silver. Silver, which has more than doubled over the past 12 months, plunged around 30% on Friday. That marked the metal’s worst one-day performance since 1980. Gold also dropped around 9%.
Bitcoin last traded near $76,000.
Wall Street also turned its attention to Nvidia as questions over the artificial intelligence loomed.
The Wall Street Journal reported, citing people familiar with the matter, that Nvidia’s plans to pour $100 billion into OpenAI had stalled, with chipmaker execs expressing doubt about the deal.
More than 100 S&P 500 companies are due to report this week, including Amazon, Alphabet and Disney. The overall reporting season has been strong thus far, but there have been some high-profile post-earnings sell-offs, including Microsoft.
Nonetheless, Deutsche Bank strategists noted this weekend that earnings growth is on track to be the strongest in four years.
Wall Street is also awaiting the release of the January U.S. jobs report, due Friday morning. Economists polled by Dow Jones expect 55,000 jobs were added last month.
Stocks are coming off a losing session, with the major benchmarks falling after President Donald Trump named Kevin Warsh as his nominee for Federal Reserve chairman. If confirmed, Warsh would replace Jerome Powell later this year.

Bankrupt retailer Saks Global is ending its “Saks on Amazon” partnership with e-commerce giant Amazon.com, a source with direct knowledge of the decision said on Friday.
The partnership was already in dire straits when Saks filed for bankruptcy in February, but the retailer had yet to say outright that it was exercising its right under Chapter 11 bankruptcy to reject the contract.
On Friday, a source said Saks will wind down its Saks on Amazon storefront so it can focus on parts of its business it sees as spurring more growth.
“The Saks on Amazon storefront saw limited brand participation,” the person said, adding that Saks feels it would be better served driving traffic to Saks.com.
Amazon did not immediately respond to a request for comment.
The partnership arose from Amazon’s $475 million investment in Saks’ business in 2024. The companies agreed to an arrangement in which Saks would sell products on Amazon, paying the e-commerce giant at least $900 million over eight years.
But comments by Amazon’s lawyer at a court hearing after Saks filed bankruptcy indicated their relationship had soured, and court battles may lie ahead.
At the hearing, the Amazon lawyer argued that Saks improperly pledged its flagship Fifth Avenue store in Manhattan as collateral for a $1.75 billion loan that is allowing it to operate while in bankruptcy. The lawyer said that property had already been collateralised to guarantee Saks’ payments to Amazon under their partnership.
The partnership was also facing pushback from Saks’ top luxury brands, who feared selling on a mass-market e-commerce site would dilute their brand, according to two sources familiar with these brands’ thinking.
It was likely the brands would use bankruptcy negotiations to push back on the deal, the people said.
By Nicholas P. Brown with additional reporting by Dietrich Knauth; Editor: Lisa Shumake
Learn more:
In the Fight for Early Payouts From Bankrupt Saks, Big Luxury Brands Have the Edge
The embattled luxury department store is ‘absolutely dependent’ on companies like Chanel, LVMH and Kering, who could ‘suffocate’ the retailer if they stopped shipping, Reuters reported.

In late January 2026, Calix reported Q4 2025 revenue of US$272.45 million and a swing to quarterly and full-year profitability, while also expanding its share repurchase authorization to US$425 million after buying back 5.06 million shares since 2022.
At the same time, Calix highlighted record non-GAAP gross margins, rapid adoption of its third-generation cloud platform with more than 300 customer migrations, and growing use cases like Zentro’s SmartMDU deployments in dense multifamily housing.
Against this backdrop of earnings strength and expanded buybacks, we’ll explore how the third-generation platform launch shapes Calix’s investment narrative.
We’ve found 12 US stocks that are forecast to pay a dividend yield of over 6% next year. See the full list for free.
To own Calix today, you have to believe in its broadband cloud platform story more than the recent share price slide. The Q4 2025 print and full-year swing to profitability, alongside record non-GAAP gross margins and US$1,000.01 million in revenue, reinforce that the third-generation platform and AI investments are becoming a larger part of the business. The expanded US$425 million buyback hints at management’s confidence, but the near 18% pullback since earnings suggests the market is focused on short term margin pressure from dual cloud costs and customer mix. Key catalysts now cluster around successful customer migrations, monetization of services like SmartMDU, and how efficiently Calix manages overlapping cloud expenses. The biggest risk is that execution hiccups on this transition keep margins and returns below what current valuation already assumes.
However, investors should not ignore how sensitive the story has become to cloud transition costs. Despite retreating, Calix’s shares might still be trading above their fair value and there could be some more downside. Discover how much.
Five Simply Wall St Community fair values for Calix span roughly US$43 to just over US$109, underlining how differently private investors assess upside after the platform-driven earnings swing and growing cloud cost risks. This spread can help you weigh the recent earnings strength against the possibility that margin pressure or execution missteps on the third generation platform shift sentiment again, with clear implications for how the market prices Calix’s transition.
Explore 5 other fair value estimates on Calix – why the stock might be worth over 2x more than the current price!
Disagree with this assessment? Create your own narrative in under 3 minutes – extraordinary investment returns rarely come from following the herd.
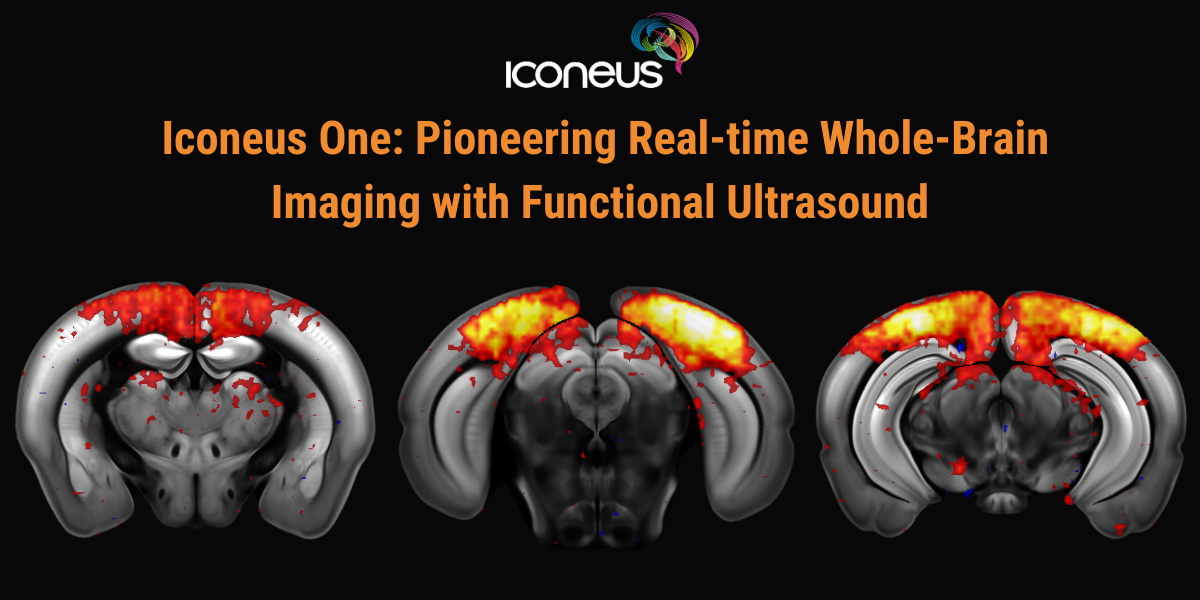
Date: February 19, 2026
Time: 7:00 AM PT, 10:00 AM ET, 4:00 PM CET
Discover how Iconeus One, the premier functional ultrasound (fUS) system for neuroscientists, is transforming brain function studies. Based on ultrafast plane-wave sonography, Iconeus One offers exceptional spatial (100 x 100 x 400 µm) and temporal (10 ms) resolution, enabling precise, real-time mapping of hemodynamic changes even in conscious subjects.
This session will highlight the diverse applications of Iconeus One in Neuroscience, including task-induced activation mapping, resting-state functional connectivity, and in vivo dynamic angiography. We’ll showcase how this cutting-edge technology contributes to our understanding of brain function and discuss its future potential with advancements like 3D solutions for whole-brain imaging and connectivity mapping in mice.
Additionally, we will cover Ultrasound Localization Microscopy (ULM), a derivative technique enabled by Iconeus One, offering super-resolution vascular imaging at the micrometer scale. ULM’s ability to visualize functional hyperemia at 6.5 µm will be demonstrated, underscoring its revolutionary impact on brain research.
Join us to learn about the transformative capabilities of Iconeus One and its role in advancing neuroimaging and Neuroscience research.
Learning Objectives:
Understand the principles and advantages of functional ultrasound (fUS) imaging
Identify the key neuroscience applications of fUS
Explore how advanced fUS platforms like Iconeus One enable cutting-edge research

The flood of Chinese exports around the world helped the economy blow past President Donald Trump’s massive tariff hikes, while Beijing touts successes in AI, EVs, robotics and other emerging technologies.
But that strength masks ongoing weakness among consumers and the property sector.
China’s trade surplus jumped 20% to $1.19 trillion in 2025, marking the world’s largest ever, as shipments surged to the European Union, Africa, Latin America and Southeast Asia.
Exports climbed 5.5% and accounted for a third of economic growth in 2025, the highest level since 1997. Imports were virtually flat, reflecting weak domestic demand and Beijing’s push to become more self-sufficient.
The record trade surplus helped GDP grow 5% last year, matching the government’s target, but the headline figure contrasted with mounting signs of broad weakness.
Growth actually slowed toward the end of the year, with GDP up 4.5% in the fourth quarter on an annual basis versus a 4.8% gain in the third quarter.
Retail sales in December inched up just 0.9%, down from 2.9% growth in October and 6.4% in May. Investment in fixed assets reversed sharply into an outright decline, collapsing 15% in December after spiking 15.7% in February.
In fact, fixed-asset investment saw its first annual drop in data going back almost three decades. That’s largely due to China’s real estate crash, which sent property investment down 17.2% last year and offset heavy spending on high-tech industries that Beijing is trying to advance.
Fitch Ratings expects China’s economy to run out of steam this year, predicting GDP growth will cool sharply to 4.1% from 5% in 2025.
“We believe domestic demand will remain constrained by sluggish consumer confidence, deflationary pressures, and investment headwinds that have broadened beyond the property-sector correction and are amplified by the local-government debt overhang,” it said in a report on Jan. 22.
But more than four years since China popped a construction bubble, about 80 million unsold or vacant homes continue to weigh on sales, prices, starts and completions.
After tinkering with attempts to revive the property sector, China has signaled it’s pivoting to a new model of development, away from the emphasis on debt-fueled investment.
“This marks the virtual abandonment of an industry that once accounted for about one-quarter of China’s gross domestic product and roughly 15% of the nonfarm workforce,” Jeremy Mark, an Atlantic Council scholar and former IMF official, wrote on Wednesday.
Many other economic problems—such as weak retail spending, deflation, as well as low consumer and business confidence—can be traced back to the free-fall in real estate, which is the main repository of life savings for hundreds of millions of households, he pointed out.
That’s as an estimated 85% of the price gains in real estate have been wiped out since 2021. As result, consumers hoard their money instead of spending it, forcing businesses to trim wages, staff and prices to remain afloat. In response, consumers pull back further.
This feedback loop has kept consumer prices flat and producer prices in negative territory. China’s overcapacity and its support for manufacturers over consumers have also stoked excess supply that drags down prices. An economy-wide price gauge shows China has been suffering from deflation for three straight years, the longest such streak since its transition to a market economy in the late 1970s.
The real estate crash is also rippling through China’s banks and local governments, as efforts to stave off more bankruptcies among developers have created “zombie” firms and mountains of debt, Mark warned.
“Even if the shockwaves from China’s collapsed property bubble eventually recede, the task of rebuilding will be daunting,” he added. “It requires not only replacing a major pillar of Chinese economic dynamism, but also the revitalization of homeowners’ deeply damaged sense of financial security.”
Economists have long urged China to rebalance its growth to a consumer-led model and away from an export- and investment-led model. President Xi Jinping’s industrial policies have even been flagged as a greater threat to the global economy than Trump’s trade war.
But last year’s reliance on exports showed that the country’s leadership remains reluctant to make the switch. While Chinese businesses have flexed their muscle as global manufacturing powerhouses, their ability to prop up the rest of the economy is in doubt.
“China’s growth model is becoming increasingly difficult to sustain,” Cornell professor Eswar Prasad wrote in a Financial Times op-ed in December.
Weak growth in employment and wages, plus the property crash and lack of confidence in the government, have weighed on consumption, he added. With little domestic demand, the only option for China’s factories is to export their output.
But Trump’s tariffs have forced exporters to look elsewhere, creating a backlash in other markets that could put up additional trade barriers and limit future growth, Prasad said.
The EU and some other large economies like Indonesia and India have already imposed some targeted tariffs on certain Chinese goods.
“As the second-largest economy in the world, China is simply too big to generate much growth from exports, and continuing to depend on export-led growth risks furthering global trade tensions,” IMF Managing Director Kristalina Georgieva warned in December.

Netflix, Inc. (NASDAQ:NFLX) is among the 12 Most Profitable NASDAQ Stocks to Buy Right Now. On January 27, Freedom Capital Markets upgraded the stock to Buy from Hold, with a price target of $104.
The adjustment followed the company’s fourth-quarter results, which beat Wall Street’s estimates for both revenue and earnings, driven mainly by an 8% increase in membership to 325 million subscribers from late 2024. Another highlight of the quarter was the streaming giant’s advertising revenue, which grew more than 2.5x to over $1.5 billion.
Based on the recommendations of 40 analysts, the stock is a Moderate Buy with a one-year average share price target of $114.79, representing an upside of 37.49% as of the close on January 30.
In other news, on January 20, Netflix, Inc. (NASDAQ:NFLX) announced that it had revised the agreement with Warner Bros. Discovery (WBD) for the latter’s pending acquisition to an all-cash transaction, as part of efforts to close the doors on Paramount’s rival offer. The takeover price would remain $27.75 per WBD share.
Netflix, Inc. (NASDAQ:NFLX) is a global entertainment company offering TV series, documentaries, movies, and games across multiple languages and genres.
While we acknowledge the potential of NFLX as an investment, we believe certain AI stocks offer greater upside potential and carry less downside risk. If you’re looking for an extremely undervalued AI stock that also stands to benefit significantly from Trump-era tariffs and the onshoring trend, see our free report on the best short-term AI stock.
READ NEXT: 10 Best Defense Stocks to Buy in the S&P 500 and 14 Best Booming Stocks to Buy Right Now.
Disclosure: None.